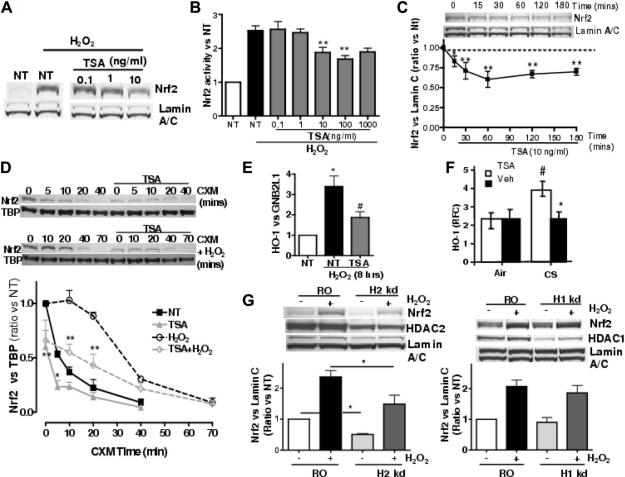
Fig. 2.
HDAC2 inhibition reduces Nrf2 protein stability. (A) BEAS2B cells were treated with TSA (0.1–10 ng/ml) for 1.5 h prior 30 min exposure to H2O2 (75 μM) and nuclear Nrf2 was measured by Western blotting. (B) Nrf2 DNA binding activity was measured in nuclear extracts stimulated with trichostatin A (TSA 0.1–1000 ng/ml) for 1.5 h prior to 30 min exposure to H2O2 (75 μM; n = 3; ∗p < 0.05, ∗∗p < 0.01 vs. H2O2). (C) Cells were stimulated with TSA (10 ng/ml) for 180 min and nuclear extracts analyzed for Nrf2 and lamin A/C expression by Western blotting. NT: non-treatment; n = 3. (D) Cells were stimulated with cycloheximide (CXM 0.5 μg/ml) in the presence or absence of 30 min pre-treatment with TSA (50 ng/ml). Cells were also stimulated with CXM in the presence of H2O2 (50 μM) with or without pre-treatment with TSA for 30 min. Nuclear extracts were analyzed by Western blot for Nrf2 and TBP as nuclear control. NT: non-treatment; n = 3. NT vs. TSA and H2O2 vs. H2O2 + TSA for each time point (∗p < 0.05, ∗∗p < 0.01). (E) Cells were treated with TSA (10 ng/ml) for 1.5 h prior to 8 h with H2O2 (75 μM). HO-1 expression was measured by QRT-PCR with GNB2L1 used as control (n = 3; ∗p < 0.05 between NT and H2O2, #p < 0.05 between TSA and H2O2). (F) Mice were treated with intranasal TSA or vehicle (Veh) for 1 h prior to 5 h to cigarette smoke (CS) or air. HO-1 was measured against β-actin using QRT-PCR, (#p < 0.05 vs. Air/Veh, ∗p < 0.05 vs. CS/Veh, (n = 5/group)). (G) Cells were transfected with HDAC2 siRNA (H2 kd), HDAC1 siRNA (H1 kd) or a random oligonucleotide (RO) for 48 h and stimulated with H2O2 (50 μM) for 30 min. Nrf2, HDAC2, HDAC1 and Lamin A/C were measured by Western blot from whole cell extracts. Values represent means ± SEM, n = 4, ∗p < 0.05.