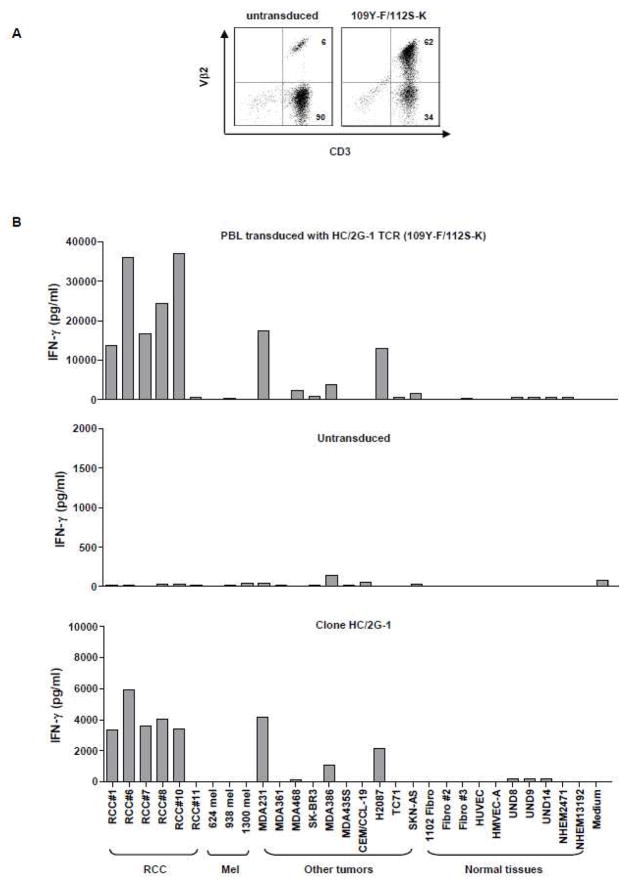
Figure 5.
Evaluation of clinical-grade retroviral supernatant derived from the modified TCR (109Y-F/112S-K). A. Vβ2 expression on PBL transduced with clinical-grade retrovirus encoding the modified TCR (109Y-F/112S-K). Retrovirally-transduced PBL were labeled with FITC-conjugated anti-CD3 and PE-conjugated anti-Vβ2 antibodies, and analyzed 3~4 days after transduction on a FACScanto II flow cytometer. Untransduced PBL were used to determine endogenous Vβ2 expression. B. Functional analysis of clinical-grade retrovirus encoding the modified TCR (109Y-F/112S-K). Retrovirally-transduced PBL were co-cultured with a panel of tumor lines and normal tissues and tested for IFN-γ production. RCC: renal tumor lines, mel: melanoma lines; MDA and SK-BR3: human breast cancer lines; CEM/CCL-19: leukemia; H2087: human non-small cell lung cancer line; TC71: Ewing’s sarcoma cell line; SKN-AS: human neuroblastoma cell line; Fibro: human fibroblasts; HUVEC and HMVEC-A: human endothelium; UND: human renal epithelium; NHEM: primary human melanocytes. Untransduced PBL and clone HC/2G-1 were included as controls.