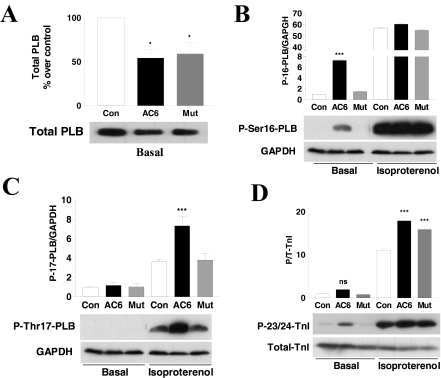
Fig. 5.
Expression and phosphorylation of PLB and cTnI proteins. A, immunodetection of total PLB protein using anti-PLB antibody in cell homogenates of adult rat cardiac myocytes. AC6 and AC6mut reduced expression of PLB. B and C, immunodetection of PLB phosphorylation at Ser16 and Thr17 using their specific antibodies in cell homogenates of adult rat cardiac myocytes unstimulated or stimulated with isoproterenol (10 μM, 24 h). AC6, but not AC6mut, increased basal PLB phosphorylation at the Ser16 site but not the Thr17 site. After isoproterenol stimulation, PLB phosphorylation at Ser16 was increased similarly in all conditions, and phosphorylation at Thr17 was increased in all conditions but was greater in AC6- than in AC6mut-infected cardiac myocytes. D, immunodetection of troponin I phosphorylation at the Ser23/24 sites using anti-phospho-cTnI antibody in cell homogenates of adult rat cardiac myocytes, unstimulated or stimulated with isoproterenol (10 μM, 24 h). AC6 and AC6mut did not increase basal cTnI phosphorylation, but isoproterenol treatment was associated with increased cTnI phosphorylation of similar degrees. Bars in all graphs show mean values from three or more experiments; error bars denote 1 S.D. (*, p < 0.05; **, p < 0.01; ***, p < 0.001).