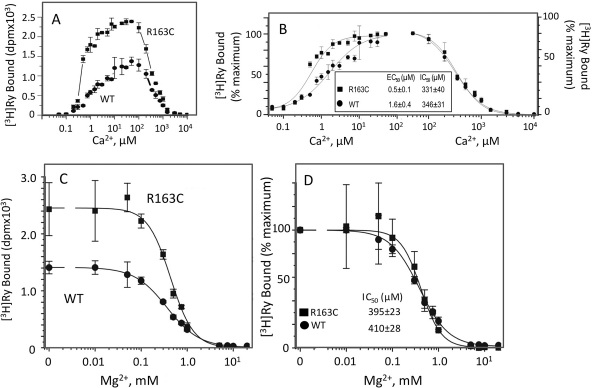
Fig. 7.
R163C exhibits altered [3H]Ry binding and Ca2+ activation, but inhibition by Ca2+ and Mg2+ remains unaltered. Equilibrium [3H]ryanodine (2 nM) binding to 100 mg/ml SR protein was performed at 37°C for 3 h in the presence of 50 nM to 10 mM Ca2+. Free Ca2+ was adjusted by EGTA according the software Bound and Determined (Voss et al., 2008). The data points are mean ± S.D. from n = 6 determinations from two independent membrane preparations from paired R163C heterozygous and WT mice. A and B, raw data and corresponding data normalized to the maximum binding within each genotype. C and D, Mg2+ (0–20 mM) inhibits equilibrium [3H]Ry binding. The free Ca2+ in the reaction mixture was buffered by EGTA. The data points are mean ± S.D. from n = 6 determinations from two independent membrane preparations from paired R163C heterozygous and WT mice.