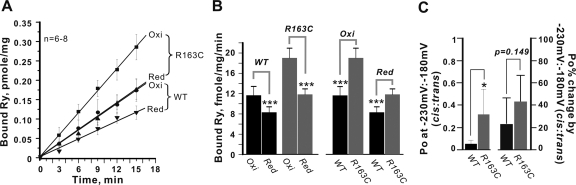
Fig. 8.
Response of R163C channels to glutathione redox potential. Reducing (Red) and oxidizing (Oxi) conditions were set in the assay buffer by addition of 5 mM GSH and 5 mM GSSG, respectively. [3H]Ry (5 nM) was added to the assay buffer to initiate the binding, and the reaction quenched at the indicated time points by filtration. The binding reaction was terminated at 0, 3, 6, 9, 12, 15 min, and the results are plotted in A. The pseudo-first order binding rates (kobs) were obtained from the linear fitting and plotted as bar graphs in B (n = 6–8 determinations). C, summarizes analysis of single-channel Po in the presence of a transmembrane redox potential set at −230 mV/−180 mV (cis/trans) for n = 6 independent R163C and WT channels reconstituted in BLM (left y-axis label). The respective changes in Po when the transmembrane redox potential were set at −230 mV/−180 mV (cis/trans) relative to the corresponding control period for WT (n = 6) and R163C (n = 6) channels are shown (right y-axis label). Mean± S.D. are shown and were not statistically different between the two genotypes (p = 0.149). Statistical analyses indicate where significant differences were found using independent Student's t tests (*, p < 0.05; ***, p < 0.001).