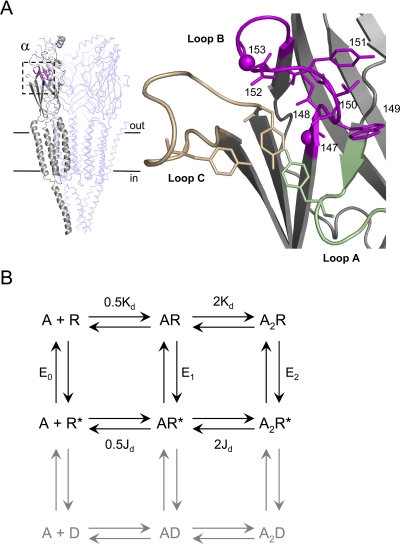
Fig. 1.
AChR structure and function. A, the transmitter binding site. Left, unliganded T. californica AChR [Protein Data Bank code 2BG9 (Unwin, 2005)]. Subunits: αg, gray; β/δ, light blue (γ and αd not displayed). Boxed area is the αg transmitter binding site. Horizontal lines approximate the location of the membrane. Right, close-up of the αg binding site. Green, loop A (αTyr93 shown); tan, loop C (αTyr190 shown); magenta, loop B (all side chains 147–153 shown, Cα atoms of αGly147 and αGly153 as spheres). B, cyclic activation scheme for the AChR. A is the agonist and the other bold letters represent stable ground states (structural ensembles represented by wells in an energy diagram). Paired arrows represent the unstable intermediates that connect the ground states. R, resting (low affinity for the agonist and low ionic conductance); R*, active (high affinity for the agonist and high ionic conductance); D, desensitized states (high affinity for the agonist and low ionic conductance) are gray. Next to the arrows are the salient equilibrium constants. E0, unliganded (spontaneous) gating; E1, monoliganded gating; E2 diliganded gating; Kd, dissociation constant for agonist binding to R; Jd, dissociation constant for agonist binding to R*. The two binding sites have approximately the same Kd and Jd for ACh and choline (Jha and Auerbach, 2010). Without an external energy source, the net energy change, R to A2R*, must be equal for the “physiological” pathway R↔ AR↔ A2R↔ A2R* and for the alternative pathway R↔ R*↔ AR*↔ A2R*. Hence, E2/Kd2 = E0/Jd2, or E2 = E0λ2.