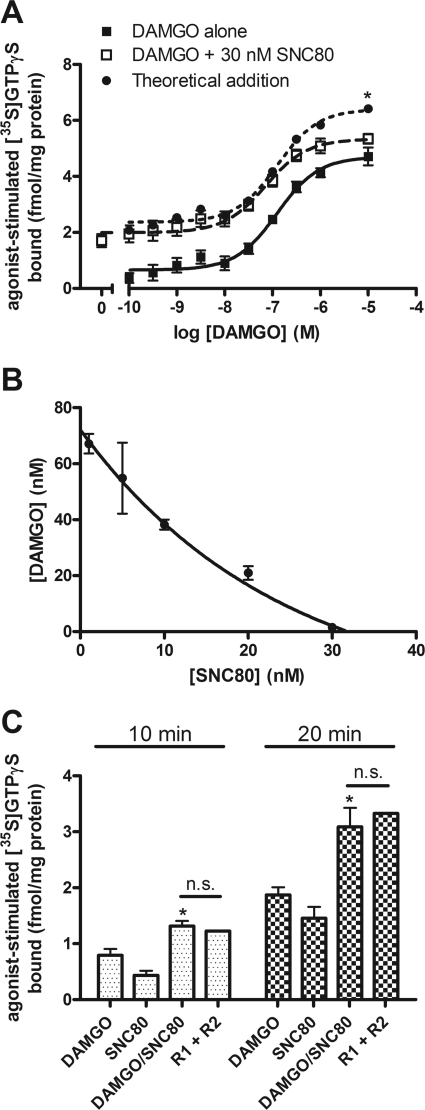
Fig. 3.
DAMGO and SNC80 activation of G protein is additive at concentrations or time points when G protein is not limiting. A, concentration-dependent stimulation of [35S]GTPγS binding in SH-SY5Y membranes after 60-min incubation with various concentrations of DAMGO alone (■) or DAMGO with 30 nM SNC80 (□). The EC50 of DAMGO is not significantly altered by the addition of 30 nM SNC80 (DAMGO alone, 121 ± 32 nM, DAMGO + 30 nM SNC80, 64 ± 12 nM; p = 0.14 by two-tailed Student's t test). Coincubation of DAMGO with 30 nM SNC80 produces additive [35S]GTPγS binding similar to the theoretical additive curve (●), which diverges only when DAMGO becomes occlusive at maximal concentrations (*, p < 0.05 at 10 μM for DAMGO + 30 nM SNC80 compared with the “theoretical addition” by two-way ANOVA with Bonferroni's post test; n = 4, in duplicate). B, isobologram for agonists with a variable potency ratio calculated as described under Materials and Methods. Stimulation of [35S]GTPγS binding by DAMGO was conducted in the presence of indicated concentrations of SNC80. Concentration combinations that produced 50% of the maximum effect of DAMGO alone are plotted from three separate experiments as mean ± S.E.M. Points on the line indicate additivity between DAMGO and SNC80. C, stimulation of [35S]GTPγS binding in SH-SY5Y membranes after incubation with 1 μM DAMGO or SNC80 alone or in combination for 10 or 20 min, before the incubation reaches steady state. At both time points, coincubation with DAMGO and SNC80 (DAMGO/SNC80) is greater than DAMGO alone (*, p < 0.05 by one-way ANOVA with Bonferroni's post test) and similar to the theoretical additive (R1 + R2) (p > 0.05 by one-way ANOVA with Bonferroni's post test; n = 2, in triplicate); n.s., not significant.