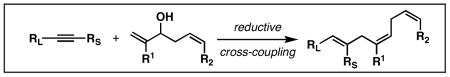
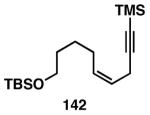
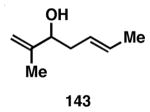
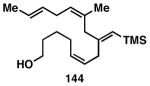
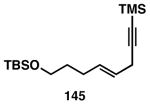
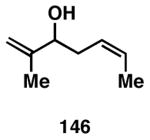
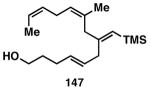
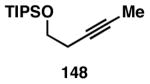
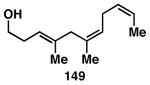
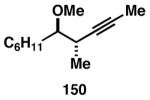
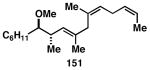
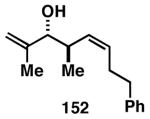
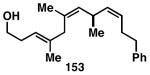
Table 3. Chemoselective reductive cross-coupling reactions of 1,5-dienes as a route to stereodefined skipped trienes.
Reaction conditions: alkyne (2-3 equiv.), Ti(Oi-Pr)4 or ClTi(Oi-Pr), c-C5H9MgCl, PhMe (−78 to −35 °C), then cool to −78 °C and add Li alkoxide of the allylic alcohol as a solution in THF (warm to 0 °C).
Yield reported is over two steps: chemoselective reductive cross-coupling and silyl deprotection with TBAF in THF.