Abstract
Severe halothane (HAL)-induced hepatotoxicity occurs in one in 6000–30,000 patients by an unknown mechanism. Female sex is a risk factor in humans and rodents. We tested the hypothesis that a sex difference in natural killer (NK) cell activity contributes to HAL-induced liver injury. HAL (15 mmol/kg, ip) treatment resulted in severe liver injury by 12 h in female, wild-type BALB/cJ mice, and the magnitude of liver injury varied with stage of the estrous cycle. Ovariectomized (OVX) mice developed only mild liver injury. Plasma interferon-gamma (IFN-γ) was elevated 10-fold in HAL-treated females compared with similarly treated male mice or with OVX female mice. IFN-γ knockout mice were resistant to severe HAL-induced liver injury. The deactivation of NK cells with anti-asialo GM1 treatment attenuated liver injury and the increase in plasma IFN-γ compared with immunoglobulin G–treated control mice. Mice with a mutated form of perforin, a protein involved in granule-mediated cytotoxicity, were protected from severe liver injury. Furthermore, HAL increased the activity of NK cells in vivo, as indicated by increased surface expression of CD69, an early activation marker. In response to HAL, NK cell receptor ligands on the surface of hepatocytes were expressed in a manner that can activate NK cells. These results confirm the sexual dimorphic hepatotoxic response to HAL in mice and suggest that IFN-γ and NK cells have essential roles in the development of severe HAL-induced hepatotoxicity.
Keywords: halothane, NK cells, inflammation, female sex, idiosyncratic adverse drug reactions
Drug-induced liver injury is the leading cause of acute liver failure in the United States (Ostapowicz et al., 2002) and the leading cause of withdrawal of U.S. Food and Drug Administration–approved drugs. Some of these hepatotoxic responses are classified as idiosyncratic adverse drug reactions (IADRs), which account for up to 20% of cases of severe liver injury requiring hospitalization (Lee, 2008). IADRs often occur in a small fraction of people undergoing drug therapy, and the reactions are typically unrelated to the pharmacology of the drug. It is likely that the mechanisms of IADRs are multifactorial, and their rarity and complex mechanisms of development are barriers to the ability to predict in preclinical testing which drugs will produce them. Information gained from mechanistic studies using animal models that reproduce the injury seen in patients would be helpful to prevent and treat IADRs.
Halothane (HAL) is an inhaled anesthetic that produces mild liver injury in one in five patients (Trowell et al., 1975) and a more severe IADR, or “HAL hepatitis,” in one in 6000–30,000 patients exposed to the drug (Mushin et al., 1971). HAL is metabolized by cytochrome P450 (CYP) 2E1 in hepatocytes to form trifluoroacetyl (TFA) chloride, which binds covalently to proteins and lipids, forming TFA adducts. Studies in which HAL hepatotoxicity in guinea pigs was ameliorated by SKF-525A, a CYP inhibitor, and exacerbated by 4-methylpyrazole, a CYP2E1 inducer, illustrate the requirement for metabolism in the development of hepatotoxicity (Farrell et al., 1996; Lunam et al., 1985). Furthermore, susceptibility to HAL hepatotoxicity in a guinea pig model correlated with the formation of liver TFA protein adducts (Bourdi et al., 2001). This suggests that TFA adducts are a prerequisite for pathogenesis; however, the mechanisms of pathogenesis for the mild and severe forms of HAL hepatotoxicity remain elusive. Hypotheses to explain the pathogenesis have been proposed; however, none has satisfactorily explained the characteristics of HAL hepatitis in human patients.
Female sex is a risk factor for IADRs from numerous drugs, including isoniazid, HAL, flucloxacillin, nitrofurantoin, and chloropromazine (Chalasani and Bjornsson, 2010). It was recently reported that females were the most at-risk group, representing 81% of patients who developed severe HAL hepatitis (Eghtesadi-Araghi et al., 2008). In mouse models of either mild or severe HAL hepatotoxicity, female mice developed liver injury at doses of HAL that produced no or minimal injury in male mice, and this disparity was not due to differences in HAL bioactivation (Dugan et al., 2010; You et al., 2006). The accumulation and activity of innate immune cells, such as natural killer (NK) cells, are influenced by sex hormones (Curran et al., 2001). In this regard, we demonstrated that HAL-treated female mice had a greater accumulation of polymorphonuclear leukocytes in the liver and a greater concentration of tumor necrosis factor-alpha (TNF-α) in plasma compared with similarly treated male mice (Dugan et al., 2010).
Residing in normal liver are several types of innate immune cells, including NK cells, natural killer T (NKT) cells, and Kupffer cells (KCs) (Nemeth et al., 2009). NK and NKT cells participate in innate immune responses through the release of rapidly induced cytokines, such as interferon-gamma (IFN-γ), and through cell-mediated cytotoxicity via Fas-Fas ligand interactions, TNF-related apoptosis-inducing ligand receptors, and/or the exocytosis of cytotoxic granules. These hepatic innate immune cells could participate in the pathogenesis of drug-induced liver injury. Indeed, evidence exists for participation of NK cells in the hepatotoxicity of other drugs such as acetaminophen (Liu and Kaplowitz, 2006; Masson et al., 2008). Injury in a model of mild HAL hepatotoxicity in mice was increased by polyinosinic:polycytidylic acid (poly(I:C)), a double-stranded RNA viral mimetic that induces the accumulation and activation of NK and NKT cells (Cheng et al., 2009). However, the role of NK and NKT cells and IFN-γ in the initiation of sexually dimorphic hepatotoxicity from HAL and other drugs associated with idiosyncratic liver injury remains unclear. Accordingly, we investigated the possibility that innate immune cells contribute to the sex disparity in HAL hepatotoxicity observed in mice.
METHODS
Materials.
HAL (2-bromo-2-chloro-1,1,1,trifluoro-ethane) and highly refined low acidity olive oil were purchased from Sigma-Aldrich (St Louis, MO). Alanine aminotransferase (ALT) reagent was purchased from Thermo Electron Corp. (Louisville, CO). Gibco liver perfusion medium, Williams' medium E, hepatocyte wash medium, and fluorescein isothiocyanate–conjugated goat antirabbit immunoglobulin G (IgG) were purchased from Invitrogen (Carlsbad, CA). PBS and Hank's balanced salt solution (HBSS), each without Ca2+ and Mg2+, were purchased from Lonza Walkersville, Inc. (Walkersville, MD). Dr Lance Pohl generously donated anti-TFA adduct rabbit serum (Satoh et al., 1985). Horseradish peroxidase–conjugated goat antirabbit antibody was purchased from Santa Cruz (Santa Cruz, CA). Lympholyte mammal density separation medium and anti-asialo GM1 Ig fraction (anti-AsGm1) were purchased from Cedarlane Laboratories Limited (Hornby, Ontario, Canada). Rabbit Ig fraction was purchased from EMD Chemicals (Gibbstown, NJ). Clodronate-containing and empty liposomes were purchased from Encapsula Nano Sciences (Nashville, TN). Allophycocyanin (APC)-conjugated antimouse retinoic acid early inducible gene-1 (Rae-1) (pan specific) was purchased from R&D systems (Minneapolis, MN). Phycoerythrin (PE)-conjugated antimouse H-2Dd, APC-Cy7 antimouse CD3e, isotype-matched antibodies, RBC lysis buffer, and fixation buffer were purchased from Biolegends (San Diego, CA). Higgins India ink was purchased from Utrecht (Canbury, NJ). Collagenase type II was purchased from Worthington Biochemical Co (Lakewood, NJ). Cell strainers of 100 μm were purchased from BD Biosciences (San Jose, CA).
Animals.
BALB/cJ, BALB/cByJ, BALB/cJ CD1d KO, BALB/cJ IFNγ KO, BALBPrf1, TLRLps-d, RAG1NULL, ovariectomized (OVX), and sham-operated (SHAM) BALB/cJ mice were purchased from Jackson Laboratory (Bar Harbor, ME). They were housed under conditions of controlled temperature and humidity and 12 h light/dark cycle. Mice were given continual access to spring water and fed a standard chow (Rodent Chow/Tek 2018; Harlan Teklad, Madison, WI) ad libitum and allowed to acclimate for a week prior to use. Eight- to 12-week-old mice were fasted for 15 h prior to HAL administration, and food was returned after HAL administration. All procedures were carried out according to the humane guidelines of the American Association for Laboratory Animal Science and the University Laboratory Animal Research Unit at Michigan State University.
Experimental protocol.
HAL solution was prepared as previously reported (Dugan et al., 2010). Unless otherwise noted, mice fasted overnight were given 15 mmol/kg HAL or olive oil, ip. Vaginal cytology was analyzed prior to HAL administration by methods described elsewhere (Goldman et al., 2007) to classify mice according to estrous cycle phase. Mice were anesthetized with isoflurane and euthanized at various times after the administration of HAL; blood was drawn from the vena cava into a syringe containing sodium citrate (final concentration of 0.76%) for preparation of plasma. Plasma ALT activity, tissue processing, histopathological analysis, and TFA western analysis were performed as previously reported (Dugan et al., 2010).
To reduce NK cell activity, mice were treated with 50 μl anti-AsGM1 or rabbit IgG, iv, 48 and 24 h before HAL administration. To deplete KCs, clodronate-containing or empty liposomes were administered 48 h before HAL administration. In some experiments, Higgins India ink (diluted 1:4 in saline) was injected iv 45 min before liver extraction to confirm KC depletion.
Isolation of hepatic lymphocytes.
The liver was isolated and gently pressed through a 100-μm mesh filter. The liver cell suspension was collected in PBS and centrifuged at 50 × g at 4°C for 3 min to remove parenchymal cells and debris. Supernatant was centrifuged at 350 × g at 4°C for 5 min, and the pellet was resuspended in RBC lysis buffer according to the manufacturer's protocol. After two washes in PBS, the cells from five animals were combined in PBS and underlaid with lympholyte mammal density separation medium. Hepatic lymphocytes were obtained by centrifugation and washed in PBS.
Immunophenotyping of hepatic lymphocytes.
After two washes in staining buffer (HBSS supplemented with 1% bovine serum albumin, 0.09% sodium azide, pH 7.6), the lymphocytes were incubated at 4°C for 30 min with antibodies to cell surface proteins, that is, either PE-conjugated CD49b mAb (clone DX5), APC-Cy7-conjugated CD3e mAb (clone 145-2C11), APC-conjugated CD69 mAB (clone H1.2F3), or the appropriate isotype control. After two washes in staining buffer, the cells were fixed in fixation buffer for 20 min at 4°C. Data were collected on a BD FACS Canto II flow cytometer and analyzed with FLO JO software (Tree Star, Inc).
Isolation of primary hepatocytes for flow cytometry.
The protocol for primary mouse hepatocyte isolation was adapted from that described by Renton (Renton, 1980). In brief, mice were anesthetized with pentobarbital (50 mg/kg, ip), and their livers were retrograde perfused with Gibco liver perfusion medium through the inferior vena cava. Williams' medium E supplemented with 0.4 mg/ml collagenase type II was then perfused to digest the liver. The liver was combed gently to disaggregate the hepatocytes into Gibco hepatocyte wash medium. Cell debris was removed by straining the cell suspension through a 100-μm cell strainer. The hepatocytes were collected after centrifugation (50 × g, 5 min) and washed twice in hepatocyte wash medium.
IFN-γ analysis.
The plasma concentration of IFN-γ was measured using a BD OpEIA mouse IFN-γ ELISA kit purchased from BD Biosciences (San Diego, CA).
Statistical analysis.
Results are presented as mean ± SEM. A Student's t-test was performed on comparisons of two groups. For comparison of more than two groups, a 1- or 2-way ANOVA was used as appropriate after data normalization. The Student-Newman-Keuls test was performed to compare means in studies in which the ANOVA indicated statistical significance. The criterion for significance was p < 0.05 for all studies.
Probability binning was performed on flow cytometry data. In brief, this algorithm divides the control sample population into bins with the same number of events and then divides the test sample along the same boundaries and calculates the Chi-square value, χ2, of the two-binned data sets. The probability binning metric, or T(x), is derived from the χ2 value and provides a quantitative measurement of the probability that the populations are different (Roederer et al., 2001; Roederer and Hardy, 2001). For our studies, T(x) > 3 was used as the criterion for significance.
RESULTS
Estrous Cycle and Ovarian Hormones Influence HAL-induced Hepatotoxicity
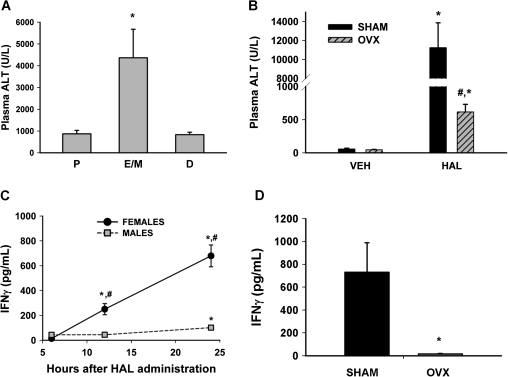
Estrous cycle phase was determined before the administration of HAL (5 mmol/kg, ip), and plasma ALT activity was evaluated 12 h later. The plasma ALT activity of HAL-treated mice in diestrus or proestrus was approximately 1000 U/l, whereas it was four to five times greater in HAL-treated mice in estrus (Fig. 1A).
FIG. 1.
HAL hepatotoxicity and the induced inflammatory response depend on ovarian hormones. (A) For each mouse, the stage of estrous cycle was determined by vaginal cytology analysis before treatment with HAL (5 mmol/kg, ip). Plasma ALT activity was measured 12 h after HAL administration (n = 3–5 per group). P, proestrus; E/M, estrus/metestrus; D, diestrus. *significantly different from other groups. (B) Plasma ALT activity was evaluated 12 h after vehicle (VEH) or HAL (15 mmol/kg, ip) administration in OVX or SHAM mice (n = 3–5 per group). *significantly different from respective VEH control; #significantly different from HAL-treated SHAM mice. (C) Plasma IFN-γ concentration was evaluated in at various times after HAL administration (n = 5–6 per group). #significantly different from time-matched male group; *significantly different from sex-matched 6 h group. (D) IFN-γ concentration was evaluated 12 h after HAL treatment in SHAM and OVX mice (n = 4 per group). *significantly different from SHAM group.
OVX and SHAM, vehicle (olive oil)-treated mice had plasma ALT activities < 100 U/l. All HAL (15 mmol/kg, ip)-treated SHAM mice developed severe liver injury as indicated by the 12 h plasma ALT activity of > 10,000 U/l and pronounced centrilobular hepatocellular necrosis on histopathological examination (Supplementary fig. 1). Similarly treated OVX mice developed mild injury with ALT activity of approximately 600 U/l (Fig. 1B).
Cytokines in HAL-Treated Mice
In an earlier publication (Dugan et al., 2010), HAL-treated female mice developed more severe liver injury with a more robust inflammatory response compared with male mice. IFN-γ is an inflammatory cytokine released from NK and NKT cells. In the present study, there was no elevation in IFN-γ concentration in plasma of vehicle (VEH)-treated mice at any time investigated (data not shown). In male mice, there was a mild elevation in plasma IFN-γ concentration 24 h after HAL administration (Fig. 1C). The plasma IFN-γ concentration in female mice was significantly increased compared with male mice at 12 and 24 h (Fig. 1C). Plasma IFN-γ concentration was significantly reduced in OVX mice compared with SHAM mice 12 h after administration of HAL (Fig. 1D).
HAL-Induced Liver Injury in IFN-γ KO Mice
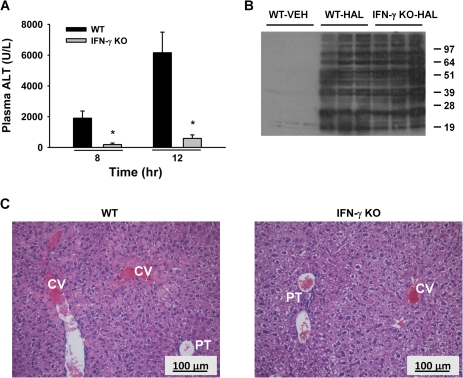
HAL exposure caused significant liver injury at 8 and 12 h in female wild-type (WT) mice as indicated by increased ALT activity (Fig. 2A). IFN-γ KO mice were protected from severe HAL-induced liver injury at both times. Histopathological examination revealed that all the WT mice treated with HAL developed severe centrilobular necrosis in the livers collected 30 h after treatment (Fig. 2C). The lesions were less severe in the livers of IFN-γ KO compared with WT mice. Several of the IFN-γ KO livers had no evidence of necrosis. In addition, livers of the protected IFN-γ KO mice showed reduced intracellular staining of hepatocytes, suggesting that glycogen accumulation was absent in the HAL-treated WT mice.
FIG. 2.
IFN-γ KO mice are protected from developing severe HAL hepatotoxicity. Female WT BALB/cJ (WT) and IFN-γ KO mice were treated with HAL (15 mmol/kg, ip), and plasma and liver samples were collected at various times. (A) Plasma ALT activity was evaluated 8 and 12 h after HAL treatment (n = 5–6 per group). *significantly different from HAL-treated WT mice. (B) Immunoblot detection of TFA protein adducts in liver homogenates (n = 3 per group). (C) Hematoxylin and eosin liver sections from HAL-treated WT and IFN-γ KO mice 30 h after treatment. Labeled in picture are central vein (CV) and portal triad (PT). Images were photographed at ×200 magnification.
A positive correlation has been reported between the severity of liver injury and the formation of TFA adducts in the livers of HAL-treated guinea pigs (Bourdi et al., 2001). There was a similar degree of TFA adduct formation in livers from HAL-treated WT and HAL-treated IFN-γ KO mice as determined by Western blot analysis (Fig. 2B). TFA adducts were not detected in the livers of VEH-treated animals.
HMGB-1 Concentration and the Role of Toll-Like Receptor 4 in HAL Hepatotoxicity
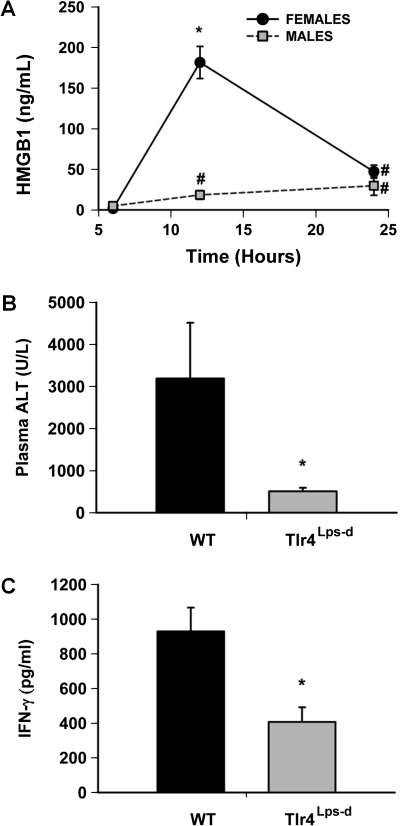
High mobility group box-1 (HMGB-1) is released from injured cells and can signal inflammatory responses by binding to pattern recognition receptors, such as toll-like receptor 4 (TLR4) (van Zoelen et al., 2009). Plasma HMGB-1 concentration did not change in VEH-treated animals at any time (data not shown). There was a slight increase in the plasma HMGB-1 concentration in HAL-treated male mice at 12 and 24 h (Fig. 3A), whereas HMGB-1 concentration markedly increased in females 12 h after HAL administration.
FIG. 3.
HMGB-1 release and the response to HAL in Tlr4Lps-d mice. (A) Plasma HMGB-1 concentration was evaluated at various times after HAL treatment (15 mmol/kg, ip) in male and female mice (n = 6 per group). VEH-treated animals had plasma HMGB1 concentrations < 5 pg/ml. #significantly different from sex-matched 6 h time point. *significantly different from time-matched male and all other female groups. (B and C) Female WT BALB/cBYJ (WT) mice and Tlr4Lps-d mice were treated with HAL (15 mmol/kg, ip). Plasma ALT activity and IFN-γ concentration were evaluated 24 h after HAL treatment (n = 4–5 per group). *significantly different from WT controls.
Mice expressing a mutant TLR4 (Tlr4Lps-d) and their WT controls (BALB/cByJ) were given HAL, and plasma and liver samples were collected 24 h later. Plasma ALT activity and IFN-γ concentration were reduced in HAL-treated Tlr4Lps-d mice compared with HAL-treated WT mice (Figs. 3B and C). Histopathologically, there were fewer necrotic cells in the liver sections from Tlr4Lps-d mice compared with those from WT mice (Supplementary fig. 2).
HAL Hepatotoxicity in KC-Depleted Mice
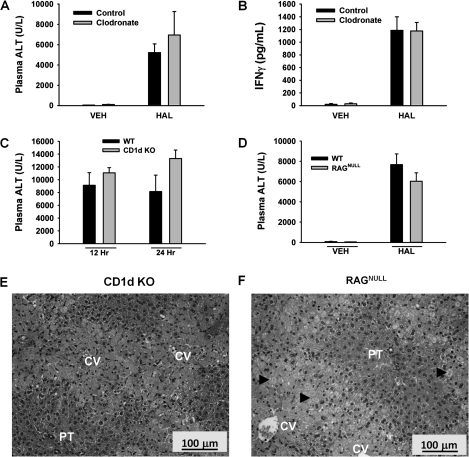
To deplete KCs, clodronate-encapsulated liposomes were injected into mice iv 48 and 24 h prior to HAL administration; empty liposomes were used as control. In a preliminary study, India ink was injected into mice to confirm effectiveness of clodronate treatment. India ink laden KCs were visible in the hepatic sinusoids of liver sections from mice pretreated with control liposomes, whereas they were not visible in clodronate-pretreated animals, confirming inhibition of KC function (Supplementary fig. 3). There was no increase in plasma ALT activity in VEH-treated mice given control liposomes or the clodronate-encapsulated liposomes. Plasma ALT activity was elevated in HAL-treated mice, and the increase was similar irrespective of clodronate inclusion in the liposomes (Fig. 4A). Plasma IFN-γ concentration was increased by HAL treatment similarly in clodronate-treated and control mice (Fig. 4B).
FIG. 4.
KC-depleted mice and CD1d KO and RAGNULL mice develop severe HAL-induced liver injury. Control- or clodronate liposome-pretreated mice were given VEH or HAL (15 mmol/kg, ip), and plasma and liver samples were collected 24 h later. Plasma ALT activity (A) and IFN-γ concentration (B) were evaluated (n = 4–6 per group). WT BALB/cJ (WT), NKT-deficient mice (CD1d KO), or T- and B cell–deficient mice (RAGNULL) were treated with HAL (15 mmol/kg, ip). (C) Plasma ALT activity was evaluated in WT and CD1d KO mice 12 and 24 h after HAL administration (n = 5 per group). (D) Plasma ALT activity was evaluated in HAL-treated WT and RAGNULL mice 12 h after HAL administration (n = 5 per group). (E and F) Hematoxylin and eosin liver sections taken 24 h after HAL treatment of CD1d KO and RAGNULL mice. Labeled in picture are central vein (CV) and portal triad (PT). Arrowheads identify areas of necrosis. Images were photographed at ×200X magnification.
HAL Hepatotoxicity in Mice Deficient in NKT Cells or Mature T and B Cells
Some findings have suggested the involvement of NKT cells in HAL hepatotoxicity in mice (Cheng et al., 2010) and of T and B cells in human HAL hepatotoxicity (Kenna et al., 1988). By using genetically engineered mice that lack NKT cells (CD1d KO mice) or mice that lack T and B cells (RAGNULL mice), we tested whether these cell types were required for the development of severe HAL-induced liver injury. HAL treatment caused similar severe liver injury in WT and CD1d KO mice as indicated by increases in plasma ALT activity that were not significantly different between the strains at either 12 or 24 h (Fig. 4C). Additionally, HAL treatment caused elevation in plasma ALT activity that was similar in WT and RAGNULL mice at 12 h (Fig. 4D). Histopathological examination of livers revealed marked centrilobular necrosis in HAL-treated CD1d KO and RAGNULL mice (Fig. 4E and F).
HAL Hepatotoxicity in Mice with Reduced NK Cell Activity
Anti-AsGM1 decreases NK cell activity in BALB/c mice (Smyth et al., 2001). CD69 is an early activation marker for T lymphocytes, B cells, NK, and NKT cells in response to stimuli. In this study, NK cells (DX5+, CD3−) were evaluated for their expression of CD69 and CD107a, a marker that the cells have undergone degranulation. NK cells from anti-AsGM1-treated mice had a decreased surface expression of CD69 (6.4%) and an increased expression of CD107a (16.2%) compared with those from IgG-treated mice (14.5 and 5.3%, respectively) (Supplementary fig. 4).
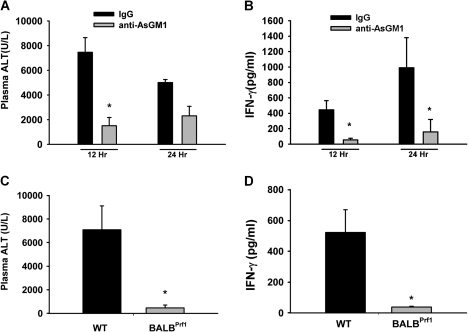
IgG-pretreated HAL-treated mice had plasma ALT activity > 7000 U/l at 12 h, indicating severe HAL hepatotoxicity, whereas after anti-AsGM1 pretreatment, HAL induced a milder hepatotoxicity with ALT activity of ∼1500 U/l (Fig. 5A). By 24 h, the ALT activity was ∼5000 and ∼2500 U/l for IgG- and anti-ASGM1-pretreated HAL-treated mice, respectively. The plasma IFN-γ concentration in HAL-treated mice was decreased markedly at both times by anti-AsGM1 pretreatment (Fig. 5B).
FIG. 5.
HAL-induced hepatotoxicity depends on NK cell activity. Mice treated with IgG or anti-AsGM1 were given HAL (15 mmol/kg, ip) as described in Methods section, and plasma samples were collected at 12 and 24 h. Plasma ALT activity (A) and IFN-γ concentration (B) (n = 4–6 per group). *significantly different from time-matched controls. Plasma ALT activity (C) and IFN-γ concentration (D) in WT and BALBPrf1 mice 12 h after HAL administration (n = 4 per group). *significantly different from WT mice.
NK cells are capable of causing granule-mediated cytotoxicity through the release of preformed cytotoxic proteins, perforin and granzyme B, which create pores in the membranes of target cells and mediate cell death (Berke, 1995). Plasma ALT activity was not increased in response to HAL in mice with defective perforin (BALBPrf1) (Fig. 5C). WT mice treated similarly developed severe liver damage. Plasma IFN-γ concentration was significantly smaller in HAL-treated BALBPrf1 mice (∼38 pg/ml) compared with similarly treated WT mice (∼500 pg/ml) (Fig. 5D).
Altered Surface Protein Expression on Hepatic NK Cells and Hepatocytes after HAL Administration In Vivo
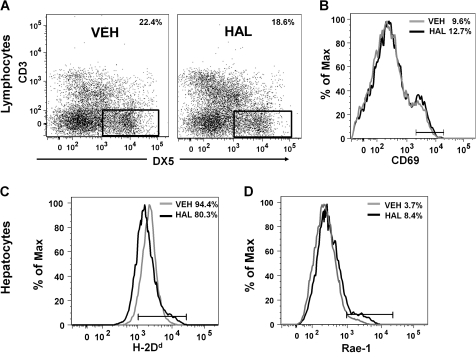
The NK cell population (i.e., DX5+, CD3− cells) was significantly decreased 8 h after HAL administration (18.6%) compared with vehicle-treated mice (22.4%) (Fig. 6A). At the same time, the number of DX5+, CD3− NK cells that were positive for CD69 increased significantly in HAL-treated (12.7%) compared with VEH-treated (9.6%) mice (Fig. 6B).
FIG. 6.
HAL treatment alters the phenotype of hepatic NK cells and hepatocytes in vivo. (A) Hepatic lymphocytes from VEH- or HAL-treated mice were pooled according to their respective groups. NK cells were gated as DX5+, CD3− as indicated in the dot plot graphs. The percentage of NK cells in the population is noted in each plot. Similar results were obtained in three independent experiments. (B) NK cell surface expression of CD69 was analyzed by flow cytometry. The gray and black histograms depict the VEH and HAL treatment groups, respectively. Experimental replicate had similar results. Hepatocytes isolated from VEH- and HAL-treated mice were immunolabeled with anti-H2Dd (C) and anti-Rae-1 (D) antibodies and analyzed by flow cytometry. The gray and black histograms depict the VEH and HAL treatment groups, respectively. Similar results were obtained in four independent replicates.
The expression of known ligands for NK receptors on hepatocytes was evaluated 8 h after HAL or VEH treatment. Administration of HAL was associated with a decrease in the percentage of hepatocytes that express H-2Dd (80.3%) compared with 94.4% in VEH-treated mice (Fig. 6C). In addition, the surface expression of Rae-1, a stress ligand for stimulatory receptors on NK cells, increased with HAL treatment (8.4%) compared with hepatocytes isolated from VEH-treated mice (3.7%) (Fig. 6D).
DISCUSSION
The exact mechanisms of pathogenesis of hepatic IADRs, and HAL hepatitis in particular, are not known. The hapten hypothesis, in which HAL's reactive metabolite, TFA chloride, modifies proteins and/or lipids to form neoantigens that trigger an adaptive immune response on re-exposure, has received wide support. Much of the support for this proposed mechanism stems from the discovery of antibodies to TFA in some patients with HAL hepatitis and the observation that multiple exposures are a risk factor; however, these features do not apply to all cases of HAL hepatitis as reviewed previously (Dugan et al., 2010). Accordingly, it remains possible that other processes could precipitate HAL hepatitis (Pohl et al., 1988).
We recently characterized a model in which human risk factors (female sex, mature age, genetics, and fasting) were imposed on mice to enable the expression of severe HAL hepatotoxicity (Dugan et al., 2010). Female sex is a risk factor for HAL hepatitis in humans as well as for the mild form of HAL-induced liver injury in mice (Dugan et al., 2010; Eghtesadi-Araghi et al., 2008; You et al., 2006). In the mouse model presented herein, HAL sensitivity in mice was ovarian hormone-dependent as indicated by loss of HAL sensitivity in OVX mice (Fig. 1B and Supplementary fig. 1). Greater HAL sensitivity was also evident in mice in estrus (Fig. 1A) in which plasma concentration of progesterone is elevated compared with proestrus (high estrogen) and diestrus (low estrogen and progesterone) (Goldman et al., 2007). Interestingly, a recent report demonstrated that mifepristrone (RU486), a potent progesterone antagonist, attenuated HAL-induced liver injury in mice (Masson et al., 2010). Knowledge of the mechanisms of sex hormone-enhanced sensitivity to HAL-induced liver injury could provide insight into the pathogenesis of other IADRs for which female sex is a predisposing factor. Moreover, if sensitivity to HAL hepatitis also varies with estrous cycle stage in humans, then risk might be decreased by scheduling general anesthesia with HAL accordingly in women.
The sex disparity in sensitivity to HAL is unlikely to be due to hormonal influences on metabolic bioactivation of HAL because male and female mice had similar hepatic TFA adduct formation (Dugan et al., 2010). Female mice that developed severe HAL hepatotoxicity had greater hepatic neutrophil accumulation and plasma TNF-α concentration (Dugan et al., 2010), suggesting that a more robust inflammatory response occurs in females than in males.
IFN-γ is a pleiotropic cytokine released mainly from cytotoxic T, NK, and NKT cells (Farrar and Schreiber, 1993). The main functions of IFN-γ are to activate macrophages and NK cells, upregulate major histocompatibility complex (MHC) class I and class II molecules, and participate in antiviral and antibacterial activities. IFN-γ also promotes liver injury in humans, as demonstrated by the finding that administration of IFN-γ to patients taking acetaminophen elevated liver enzyme activity in serum (Kellokumpu-Lehtinen et al., 1989). Exogenous IFN-γ administration caused apoptosis in a hepatocyte cell line (HepG2.2.15), and mice genetically engineered to overexpress IFN-γ spontaneously developed hepatitis (Shi and Guan, 2009; Toyonaga et al., 1994). IFN-γ has also been implicated in the pathogenesis of liver injury in concanavalin A and acetaminophen animal models (Ishida et al., 2002; Tagawa et al., 1997). In the present study, IFN-γ KO mice were protected from severe HAL-induced liver injury (Fig. 2A and C). Although IFN-γ has been reported to decrease the activity of some cytochrome P450 enzymes (Renton et al., 1978), the formation of TFA adducts was similar in WT and IFN-γ KO mice (Fig. 2B), suggesting no difference in HAL bioactivation. There are several proposed mechanisms by which IFN-γ can enhance liver injury, including through the production of inflammatory mediators, the recruitment of inflammatory cells, and the suppression of liver regeneration (Gao, 2005); however, the mechanism by which IFN-γ contributes to HAL-induced hepatotoxicity is not known.
TLRs are pattern recognition receptors that, on ligation, activate the innate immune response (Medzhitov and Janeway, 2000). TLR4 binds to microbial ligands, such as lipopolysaccharide, and to endogenous ligands, such as heat shock proteins and HMGB-1, to initiate inflammatory cascades. TLR4 signaling is also involved in HAL-induced liver injury because Tlr4Lps-d mice have an attenuated response (Fig. 3). Because HMGB-1 concentration in plasma was elevated by HAL treatment and the elevation was greater in females than in refractory males (Fig. 3), it is possible that HMGB-1 acts as a TLR4 agonist to enhance toxicity in HAL-treated female mice. The source of HMGB-1 is unknown, but it could arise from a direct effect of reactive TFA on hepatocytes.
NK cells participate in stress surveillance to destroy tumor cells, infected or aberrant host cells, foreign cells, and pathogens. In humans, NK cells (CD56+, CD3−) constitute 30–50% of hepatic lymphocytes (Nemeth et al., 2009). Similarly, a large number of hepatic NK cells were found in BALB/cJ mice, with 22% of hepatic lymphocytes immunophenotyped as DX5+, CD3− NK cells (Fig. 6A). Other reports indicate that 5–10% of the hepatic lymphocytes were NK cells in female, C57BL/6 mice (Gregoire et al., 2007), a strain insensitive to HAL hepatitis (Dugan et al., 2010). Thus, the disparity in resident NK cells in livers of BALB/c mice compared with C57/BL6 mice could contribute to the differences in immune response between the two strains (Heinzel et al., 1989), and this could in turn underlie the difference in susceptibility to HAL hepatotoxicity. The observation that BALB/c mice and humans have similar proportions of hepatic NK cells could indicate that this strain is appropriate to model human NK cell–mediated liver pathology.
HAL administration decreased the numbers of DX5+, CD3− NK cells in the hepatic lymphocyte pool at 8 (Fig. 6A) and 24 h (data not shown). It is possible that this decrease resulted from increased sequestration of NK cells within the hepatic parenchyma that excludes them from the isolation procedure or that exposure to HAL alters the surface expression of DX5. The percentage of DX5+, CD3− NK cells extracted from HAL-treated mice that were positive for CD69 was increased compared with NK cells isolated from vehicle-treated mice (Fig. 6B). This suggests that HAL treatment can enhance hepatic NK cell activity in vivo.
Anti-AsGM1 treatment diminished antitumor activity attributed to NK cells (Habu et al., 1981) and decreased NK cell–mediated cytotoxicity in vitro (Kasai et al., 1980; Masson et al., 2008). In the present study, anti-AsGM1 treatment induced a phenotype shift in the expression of CD69 and CD107a, suggesting deactivation and degranulation of NK cells (Supplementary fig. 4B). Anti-AsGM1 also abrogated HAL-induced plasma IFN-γ increases compared with IgG-treated control mice and diminished liver injury at 12 h (Fig. 5A and B). These results suggest that IFN-γ release was NK cell dependent. Additional support for a role of NK cells in HAL-induced liver injury came from the observations that mice with defective perforin were protected from HAL hepatotoxicity (Fig. 5C) and that the increase in plasma IFN-γ was reduced in these mice compared with WT mice (Fig. 5D). The finding presented here with anti-AsGM1 contrasts with another report in which anti-AsGM1 did not affect HAL hepatotoxicity (Cheng et al., 2010). Some explanations for the disparity in these results lie in the anti-AsGM1 dosing regimen (one administration at 48-h in the study of Cheng et al. and two administrations in studies presented here) and the time at which liver injury was evaluated (15 and 12 h, respectively). With respect to the latter, it is noteworthy that, by 24 h in our studies, plasma ALT activity was returning toward baseline in IgG-treated mice but had not changed in anti-AsGM1-treated mice, so that the values were not different between the groups.
HAL hepatotoxicity depends on female sex hormones (Fig. 1B) and NK cells (Fig. 5)—might there be a connection? This question was not addressed experimentally in these studies, but it is known that NK cells, as well as other leukocytes, express estrogen and progesterone receptors (Gilliver, 2010) and that sex hormones can modulate NK cell activity. For example, 17β-estradiol administration increased the intracellular IFN-γ pool in NK cells stimulated with phorbol myristate acetate and ionomycin (Hao et al., 2007). In addition, sexually dimorphic NK responses have been reported in humans and experimental animals. Females more rapidly reject skin allografts compared with males (Graff et al., 1969; Hirasawa and Enosawa, 1989) and suffer a smaller incidence of hepatocellular carcinoma (Bosch et al., 2005); both these events involve immune surveillance mediated by NK cells. Moreover, IFN-γ, which is released by NK cells, and HMGB-1 were elevated in HAL-treated intact female mice compared with HAL-treated male mice or with HAL-treated OVX mice (Figs. 1C and D and 3A). This suggests that IFN-γ and HMGB-1 might be part of a sexually dimorphic innate immune response in HAL-treated mice. These observations raise the possibility that interaction of NK cells and ovarian hormones contribute to HAL hepatotoxicity in this model.
Pharmacological and genetic approaches were used to evaluate the necessity of KCs, NKT, T, and B cells in the pathogenesis of severe HAL-induced liver injury. KCs are capable of promoting early IFN-γ release; however, it has been reported (Cheng et al., 2009) and confirmed here (Fig. 4A) that KCs are not necessary for the development of HAL hepatotoxicity in mice. Furthermore, KCs do not contribute significantly to HAL-induced IFN-γ accumulation (Fig. 4B). Cotreatment with poly(I:C), a double-stranded RNA mimic and ligand for TLR3, exacerbated HAL hepatotoxicity in a KC-dependent manner (Cheng et al., 2009). Therefore, KCs are not essential for the initiation of HAL hepatotoxicity but can be activated to exacerbate liver injury. The role of NKT cells is less clear. CD1d KO mice deficient in NKT cells develop severe HAL-induced liver injury (Fig. 4C). This finding is in contrast to a report that CD1d KO mice were protected from HAL-induced liver injury (Cheng et al., 2010). It is possible that differences in the two mouse models, such as the imposition of fasting and a different method of HAL preparation, could have led to disparate results. Our finding that RAGNULL mice deficient in mature T and B cells are capable of HAL bioactivation (Supplementary fig. 5) and develop severe HAL-induced liver injury (Fig. 4D) further confirms that cells with the RAG gene (T, B, and NKT cells) are not required for injury. However, these cells might upregulate the activity of cells that mediate HAL-induced liver injury. For example, NK cells have Fc receptors that bind to Ig, suggesting a mechanism whereby plasma antibodies could modulate NK cell activity. Interestingly, NK cells from nude mice, which also lack T and B cells, required preactivation with poly(I:C) to cause virus-induced liver injury, whereas prior activation was not required in WT mice (Liu et al., 2000). This underscores the highly integrated nature of the two arms of the immune system and also suggests that elevated Ig levels, which are considered to be diagnostic of a humoral-mediated toxicity, might enhance NK cell activity.
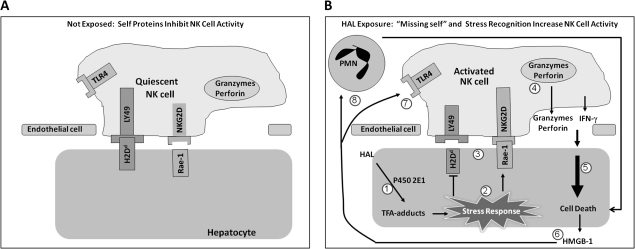
Because the bioactivation of HAL occurs primarily within hepatocytes, it is possible that the resulting TFA adducts induce an intracellular stress response that targets affected cells for cytotoxic killing by surveillant immune cells (Fig. 7). This is an attractive concept because hepatic NK cells physically interact with hepatic parenchymal cells (Wisse et al., 1976). One way that a cell can communicate its stressed status to adherent immune effector cells such as NK cells is by changing the expression of MHC class I and MHC class I–like molecules on its plasma membrane (Gleimer and Parham, 2003). MHC class I–like molecules expressed on the surface of epithelial cells signal intracellular distress to surveillent immune cells. In mice, MHC class I–like molecules, such as Rae-1 and Mult-2, expressed during cell stress, bind to NKG2D stimulatory receptors on NK cells (Cerwenka et al., 2000), and induce NK cell activity (Jinushi et al., 2003). HAL-treated hepatocytes had increased Rae-1 expression compared with hepatocytes from vehicle-treated mice (Fig. 6D). MHC class I molecules are genetically similar to MHC class I–like proteins, but their function has diverged to include antigen presentation in cell-mediated immunity. Additionally, MHC class I molecules expressed on epithelial cells (e.g., hepatocytes) downregulate NK cell activity (Jamieson et al., 2002) and the loss of epithelial MHC class I molecules activates NK cells (Ljunggren and Karre, 1986). This is known as the “missing self-hypothesis.” After HAL treatment, there were fewer hepatocytes expressing H-2Dd, a murine MHC class I molecule (Fig. 6A). Therefore, the direct interaction and signaling of HAL-stressed hepatocytes with cytotoxic NK cells is one way in which the innate immune system might lead to severe HAL-induced hepatotoxicity in females.
FIG. 7.
Proposed mechanism of innate immune-mediated severe HAL-induced liver injury. (A) In the absence of stress stimulation, self-proteins such as H2Dd are expressed on the plasma membrane of hepatocytes and RAE-1 is not; this condition keeps NK cells quiescent. (B) When hepatocytes are exposed to HAL, intracellular TFA adducts form (1). This induces a stress response in hepatocytes (2) that alters the surface NK receptor ligands (3) and activates NK cells. Activated NK cells release IFN-γ as well as the contents of cytotoxic granules (4), such as perforin and granzyme B, which contribute to hepatocellular necrosis (5). Damaged hepatocytes release endogenous danger signals, such as HMGB-1 (6). These endogenous danger signals are ligands for TLR4 (7), and the resultant signals are involved in a positive feedback loop that further activates NK cells as well as recruits polymorphonuclear leukocytes (PMNs) (Scaffidi et al., 2002) that participate (8) in the progression of injury (Dugan et al., 2010).
The results of this study significantly enhance our understanding of the effector cells that contribute to the sexually dimorphic HAL response in mice. Ovarian hormones predispose female mice to severe HAL hepatotoxicity mediated through IFN-γ and probably HMGB-1. NK cells contribute to the onset of pathogenesis in an IFN-γ- and perforin-dependent manner. IFN-γ and the phenotype of circulating NK cells might ultimately provide serum biomarkers and potential therapeutic screens for individuals sensitive to HAL hepatitis and possibly other drug-induced hepatotoxicities.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
This work was supported by the National Institutes of Health (DK061315). C.M.D. was supported by the National Institute of Environmental Health Sciences Training Grant (T32 ES007255).
References
- Berke G. The CTL's kiss of death. Cell. 1995;81:9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- Bosch FX, Ribes J, Cleries R, Diaz M. Epidemiology of hepatocellular carcinoma. Clin. Liver Dis. 2005;9:191–211. doi: 10.1016/j.cld.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Bourdi M, Amouzadeh H, Rushmore T, Martin J, Pohl L. Halothane-induced liver injury in outbred guinea pigs: role of trifluoracetylated protein adducts in animal susceptibility. Chemical Res. Toxicol. 2001;14:362–370. doi: 10.1021/tx000244x. [DOI] [PubMed] [Google Scholar]
- Cerwenka A, Bakker AB, McClanahan T, Wagner J, Wu J, Phillips JH, Lanier LL. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity. 2000;12:721–727. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- Chalasani N, Bjornsson E. Risk factors for idiosyncratic drug-induced liver injury. Gastroenterology. 2010;138:2246–2259. doi: 10.1053/j.gastro.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, You Q, Yin H, Holt M, Franklin C, Ju C. Effect of polyI: C cotreatment on halothane-induced liver injury in mice. Hepatology. 2009;49:215–226. doi: 10.1002/hep.22585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, You Q, Yin H, Holt MP, Ju C. Involvement of natural killer T cells in halothane-induced liver injury in mice. Biochem. Pharmacol. 2010;80:255–261. doi: 10.1016/j.bcp.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran EM, Berghaus LJ, Vernetti NJ, Saporita AJ, Lubahn DB, Estes DM. Natural killer cells express estrogen receptor-alpha and estrogen receptor-beta and can respond to estrogen via a non-estrogen receptor-alpha-mediated pathway. Cell Immunol. 2001;214(1):12–20. doi: 10.1006/cimm.2002.1886. [DOI] [PubMed] [Google Scholar]
- Dugan CM, MacDonald AE, Roth RA, Ganey PE. A mouse model of severe halothane hepatitis based on human risk factors. J. Pharmacol. Exp. Ther. 2010;333:364–372. doi: 10.1124/jpet.109.164541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghtesadi-Araghi P, Sohrabpour A, Vahedi H, Saberi-Firoozi M. Halothane hepatitis in Iran: a review of 59 cases. World J. Gastroenterol. 2008;14:5322–5326. doi: 10.3748/wjg.14.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar MA, Schreiber RD. The molecular cell biology of interferon-gamma and its receptor. Annu. Rev. Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- Farrell GC, Frost L, Tapner M, Field J, Weltman M, Mahoney J. Halothane-induced liver injury in guinea-pigs: importance of cytochrome P450 enzyme activity and hepatic blood flow. J. Gastroenterol. Hepatol. 1996;11:594–601. doi: 10.1111/j.1440-1746.1996.tb01708.x. [DOI] [PubMed] [Google Scholar]
- Gao B. Cytokines, STATs and liver disease. Cell Mol. Immunol. 2005;2:92–100. [PubMed] [Google Scholar]
- Gilliver SC. Sex steroids as inflammatory regulators. J. Steroid Biochem. Mol. Biol. 2010;120(2–3):105–115. doi: 10.1016/j.jsbmb.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Gleimer M, Parham P. Stress management: MHC class I and class I-like molecules as reporters of cellular stress. Immunity. 2003;19:469–477. doi: 10.1016/s1074-7613(03)00272-3. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res. B Dev. Reprod. Toxicol. 2007;80(2):84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Graff RJ, Lappe MA, Snell GD. The influence of the gonads and adrenal glands on the immune response to skin grafts. Transplantation. 1969;7:105–111. doi: 10.1097/00007890-196902000-00003. [DOI] [PubMed] [Google Scholar]
- Gregoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, Walzer T. The trafficking of natural killer cells. Immunol. Rev. 2007;220:169–182. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habu S, Fukui H, Shimamura K, Kasai M, Nagai Y, Okumura K, Tamaoki N. In vivo effects of anti-asialo GM1. I. Reduction of NK activity and enhancement of transplanted tumor growth in nude mice. J. Immunol. 1981;127:34–38. [PubMed] [Google Scholar]
- Hao S, Zhao J, Zhou J, Zhao S, Hu Y, Hou Y. Modulation of 17beta-estradiol on the number and cytotoxicity of NK cells in vivo related to MCM and activating receptors. Int. Immunopharmacol. 2007;7(13):1765–1775. doi: 10.1016/j.intimp.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J. Exp. Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa K, Enosawa S. Sex differences in the prolongation of allogeneic skin graft survival in rats treated with cyclosporin A: effects of orchiectomy and pregnancy. Transplant. Proc. 1989;21(1 Pt 1):881–884. [PubMed] [Google Scholar]
- Ishida Y, Kondo T, Ohshima T, Fujiwara H, Iwakura Y, Mukaida N. A pivotal involvement of IFN-gamma in the pathogenesis of acetaminophen-induced acute liver injury. FASEB J. 2002;16:1227–1236. doi: 10.1096/fj.02-0046com. [DOI] [PubMed] [Google Scholar]
- Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17:19–29. doi: 10.1016/s1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- Jinushi M, Takehara T, Tatsumi T, Kanto T, Groh V, Spies T, Kimura R, Miyagi T, Mochizuki K, Sasaki Y, Hayashi N. Expression and role of MICA and MICB in human hepatocellular carcinomas and their regulation by retinoic acid. Int. J. Cancer. 2003;104(3):354–361. doi: 10.1002/ijc.10966. [DOI] [PubMed] [Google Scholar]
- Kasai M, Iwamori M, Nagai Y, Okumura K, Tada T. A glycolipid on the surface of mouse natural killer cells. Eur. J. Immunol. 1980;10:175–180. doi: 10.1002/eji.1830100304. [DOI] [PubMed] [Google Scholar]
- Kellokumpu-Lehtinen P, Iisalo E, Nordman E. Hepatotoxicity of paracetamol in combination with interferon and vinblastine. Lancet. 1989;1:1143. doi: 10.1016/s0140-6736(89)92424-0. [DOI] [PubMed] [Google Scholar]
- Kenna JG, Satoh H, Christ DD, Pohl LR. Metabolic basis for a drug hypersensitivity: antibodies in sera from patients with halothane hepatitis recognize liver neoantigens that contain the trifluoroacetyl group derived from halothane. J. Pharmacol. Exp. Ther. 1988;245:1103–1109. [PubMed] [Google Scholar]
- Lee WM. Etiologies of acute liver failure. Semin. Liver Dis. 2008;28(2):142–152. doi: 10.1055/s-2008-1073114. [DOI] [PubMed] [Google Scholar]
- Liu ZX, Govindarajan S, Okamoto S, Dennert G. NK cells cause liver injury and facilitate the induction of T cell-mediated immunity to a viral liver infection. J. Immunol. 2000;164:6480–6486. doi: 10.4049/jimmunol.164.12.6480. [DOI] [PubMed] [Google Scholar]
- Liu ZX, Kaplowitz N. Role of innate immunity in acetaminophen-induced hepatotoxicity. Expert. Opin. Drug Metab. Toxicol. 2006;2:493–503. doi: 10.1517/17425255.2.4.493. [DOI] [PubMed] [Google Scholar]
- Ljunggren HG, Karre K. Experimental strategies and interpretations in the analysis of changes in MHC gene expression during tumour progression. Opposing influences of T cell and natural killer mediated resistance? J. Immunogenet. 1986;13(2–3):141–151. doi: 10.1111/j.1744-313x.1986.tb01095.x. [DOI] [PubMed] [Google Scholar]
- Lunam CA, Cousins MJ, Hall PD. Guinea-pig model of halothane-associated hepatotoxicity in the absence of enzyme induction and hypoxia. J. Pharmacol. Exp. Ther. 1985;232:802–809. [PubMed] [Google Scholar]
- Masson MJ, Carpenter LD, Graf ML, Pohl LR. Pathogenic role of natural killer T and natural killer cells in acetaminophen-induced liver injury in mice is dependent on the presence of dimethyl sulfoxide. Hepatology. 2008;48:889–897. doi: 10.1002/hep.22400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson MJ, Collins LA, Carpenter LD, Graf ML, Ryan PM, Bourdi M, Pohl LR. Pathologic role of stressed-induced glucocorticoids in drug-induced liver injury in mice. Biochem. Biophys. Res. Commun. 2010;397:453–458. doi: 10.1016/j.bbrc.2010.05.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Janeway C., Jr Innate immune recognition: mechanisms and pathways. Immunol. Rev. 2000;173:89–97. doi: 10.1034/j.1600-065x.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- Mushin WW, Rosen M, Bowen DJ, Campbell H. Post-halothane jaundice in relation to previous administration of halothane. BMJ. 1971;3:18–22. doi: 10.1136/bmj.3.5765.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E, Baird AW, O'Farrelly C. Microanatomy of the liver immune system. Semin. Immunopathol. 2009;31:333–343. doi: 10.1007/s00281-009-0173-4. [DOI] [PubMed] [Google Scholar]
- Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann. Intern. Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- Pohl L R, Satoh H, Christ D, Kenna J. The immunologic and metabolic basis of drug hypersensitivities. Annu. Rev. Pharmacol. Toxicol. 1988;28:367–387. doi: 10.1146/annurev.pa.28.040188.002055. [DOI] [PubMed] [Google Scholar]
- Renton KW. Methyl red azo-reductase and its induction by 3-methylcholanthrene in the liver by different species. Xenobiotica. 1980;10:243–246. doi: 10.3109/00498258009033751. [DOI] [PubMed] [Google Scholar]
- Renton KW, Deloria LB, Mannering GJ. Effects of polyribonoinosinic acid polyribocytidylic acid and a mouse interferon preparation on cytochrome P-450-dependent monooxygenase systems in cultures of primary mouse hepatocytes. Mol. Pharmacol. 1978;14:672–681. [PubMed] [Google Scholar]
- Roederer M, Hardy RR. Frequency difference gating: a multivariate method for identifying subsets that differ between samples. Cytometry. 2001;45(1):56–64. doi: 10.1002/1097-0320(20010901)45:1<56::aid-cyto1144>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Roederer M, Moore W, Treister A, Hardy RR, Herzenberg LA. Probability binning comparison: a metric for quantitating multivariate distribution differences. Cytometry. 2001;45(1):47–55. doi: 10.1002/1097-0320(20010901)45:1<47::aid-cyto1143>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Satoh H, Fukuda Y, Anderson D, Ferrans V, Gillette J, Pohl L. Immunological studies on the mechanism of halothane-induced hepatotoxicity: immunohistochemical evidence of trifluoroacetylated hepatocytes. J. Pharmacol. Exp. Ther. 1985;233:857–862. [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Shi H, Guan SH. Increased apoptosis in HepG2.2.15 cells with hepatitis B virus expression by synergistic induction of interferon-gamma and tumour necrosis factor-alpha. Liver Int. 2009;29:349–355. doi: 10.1111/j.1478-3231.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Cretney E, Takeda K, Wiltrout RH, Sedger LM, Kayagaki N, Yagita H, Okumura K. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) contributes to interferon gamma-dependent natural killer cell protection from tumor metastasis. J. Exp. Med. 2001;193:661–670. doi: 10.1084/jem.193.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa Y, Sekikawa K, Iwakura Y. Suppression of concanavalin A-induced hepatitis in IFN-gamma(-/-) mice, but not in TNF-alpha(-/-) mice: role for IFN-gamma in activating apoptosis of hepatocytes. J. Immunol. 1997;159:1418–1428. [PubMed] [Google Scholar]
- Toyonaga T, Hino O, Sugai S, Wakasugi S, Abe K, Shichiri M, Yamamura K. Chronic active hepatitis in transgenic mice expressing interferon-gamma in the liver. Proc. Natl. Acad. Sci. U.S.A. 1994;91:614–618. doi: 10.1073/pnas.91.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowell J, Peto R, Smith A. Controlled trial of repeated halothane anaesthetics in patients with carcinoma of the uterine cervix treated with radium. Lancet. 1975;305:821–824. [PubMed] [Google Scholar]
- van Zoelen MA, Yang H, Florquin S, Meijers JC, Akira S, Arnold B, Nawroth PP, Bierhaus A, Tracey KJ, Poll T. Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock. 2009;31(3):280–284. doi: 10.1097/SHK.0b013e318186262d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse E, van't Noordende JM, van der Meulen J, Daems WT. The pit cell: description of a new type of cell occurring in rat liver sinusoids and peripheral blood. Cell Tissue Res. 1976;173(4):423–435. doi: 10.1007/BF00224305. [DOI] [PubMed] [Google Scholar]
- You Q, Cheng L, Reilly T, Wegmann D, Ju C. Role of neutrophils in a mouse model of halothane-induced liver injury. Hepatology. 2006;44:1421–1431. doi: 10.1002/hep.21425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.