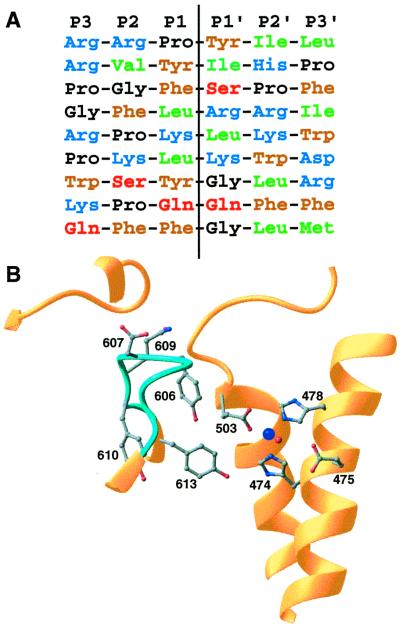
Figure 4.
Sequence selectivity. (A) Aligned sequences for known neurolysin cleavage sites. The sites listed are, top to bottom, from the neuropeptides neurotensin, angiotensin II, bradykinin, dynorphin A (residues 1–8), dynorphin A (residues 1–17), a second site in dynorphin A (residues 1–17), luteninizing hormone-releasing hormone, substance P, and a second site in substance P (8, 35). Residue types are indicated by different colors (blue = basic, brown = aromatic, green = aliphatic, black = proline or glycine, red = polar), and the hydrolysis position is indicated by the vertical line. (B) Details of the active site and nearby disordered loop (light blue; residues 600–612). The zinc cofactor is shown in dark blue and the catalytic water in red. Some side chains from residues in the mobile loop and active site are shown.