Fig. 3.
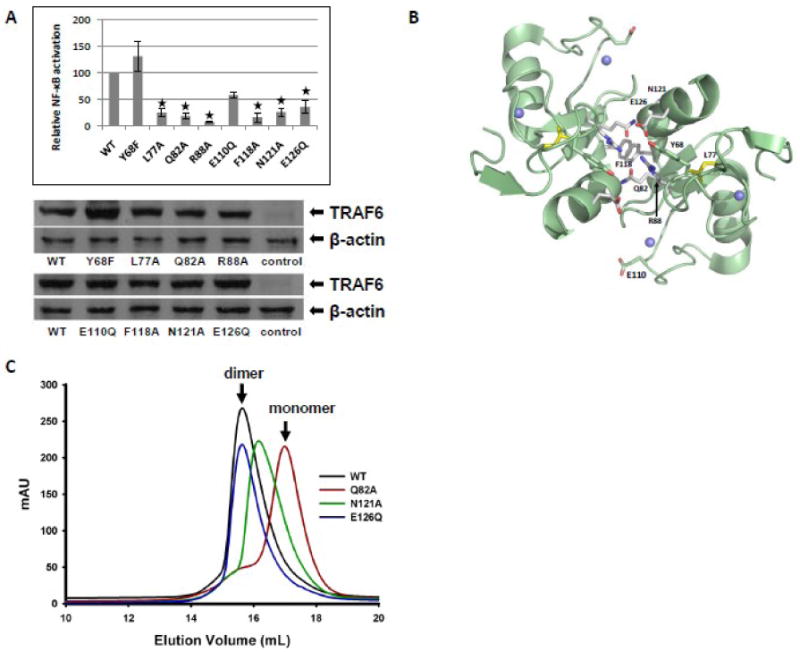
NF-κB activation and dimerization potential of RING domain mutants of TRAF6. (A) Relative activity of a NF-κB-responsive luciferase reporter in response to expression of FLAG-tagged wild type or the indicated TRAF6 mutants in HEK293T cells (upper panel). The results are the means ± SE of relative luciferase activity from at least three independent experiments. The asterisks designate the TRAF6 mutants that show a statistically significant difference in their ability to activate NF-κB relative to wild type TRAF6. Representative Western blots showing the expression of FLAG-tagged wild type or mutated TRAF6 isoforms and β-actin in cell lysates analyzed in A (lower panels). (B) Molecular modeling of the RING domain of TRAF6. A dimer of the RING domain is shown. Residues that were mutated are shown as sticks. Grey: residues on RING dimer interface. (C) Superimposed gel filtration profiles of wild-type (WT) and RING domain mutants of TRAF6 (50-211).
