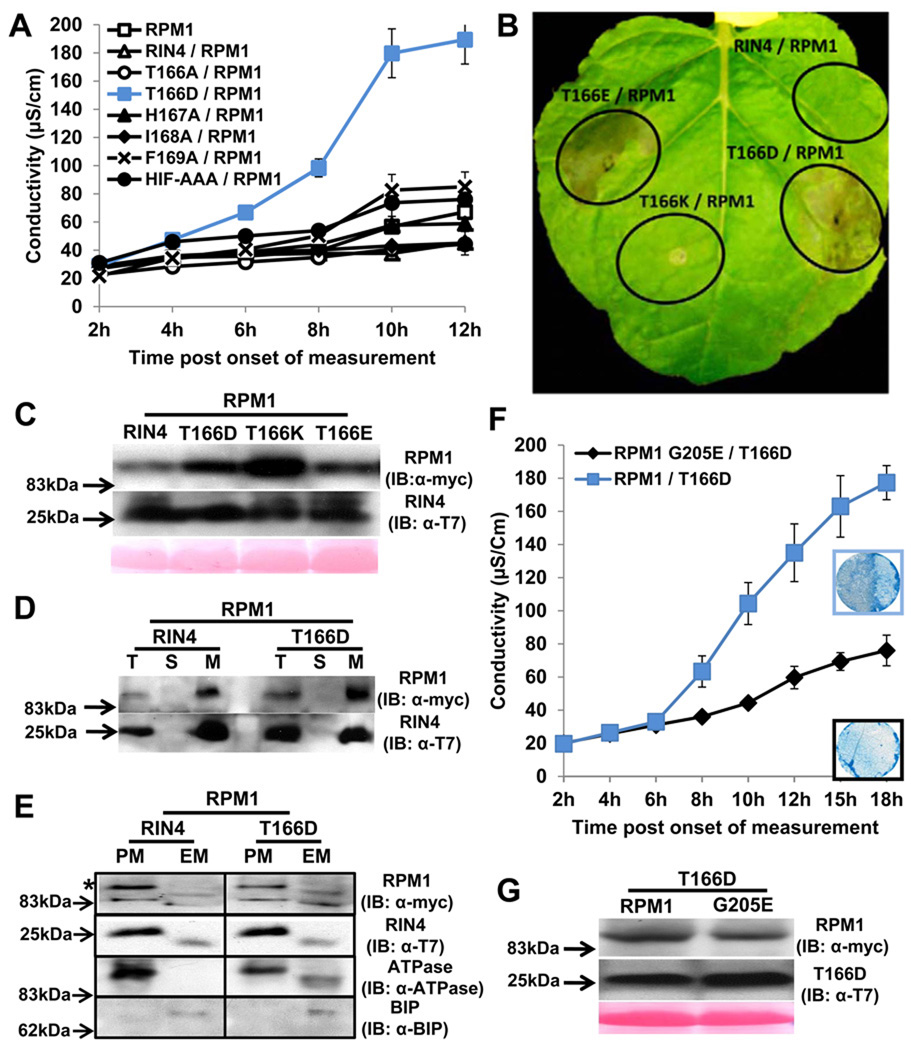
Figure 3. RIN4 T166D activity is dependent on RPM1 P-loop function in Nicotiana benthamiana.
(A) The phosphomimic RIN4 T166D mutant drives effector-independent RPM1-mediated HR. Conductivity measurements were performed with N. benthamiana leaves infiltrated with Agrobacterium C58C1 strains expressing RIN4 BBS mutants (OD600=0.4) and RPM1:myc (OD600=0.4). The measurements began two days post infiltration. Repeated three times with similar result. The error bars represent 2x SE.
(B) Phenotypes of RIN4 T166 derivatives. RIN4 T166D, RIN4 T166E and RIN4 T166K driven by the RIN4 native promoter were co-infiltrated as in (A). Photo was taken 3 days after co-infiltration.
(C) Expression of RPM1 and RIN4 T166 derivatives. RPM1 and RIN4 T166 derivatives were expressed, and variation does not account for the observed phenotypes. Immunoblots with α-myc and α-T7 were performed with 2 day-old-samples post infiltration.
(D) The RIN4 phosphomimic T166D is localized to a microsomal fraction. N. benthamiana leaves were co-infiltrated as in (A). Proteins were extracted from leaf tissues at the onset of HR from T166D/RPM1 co-infiltration, which corresponded to 8 hours in the conductivity experiment in (A). Repeated twice. Total (T), soluble (S) and microsomal (M) fractions were loaded at a 1:1:5 ratio, followed by immunoblotting with α-T7 and α-myc to detect RIN4 and RPM1, respectively.
(E) Two-phase partitioning of RIN4 and RPM1. RIN4 and RIN4 T166D mutant were co-infiltrated with RPM1 as described in (D). The microsomal extraction was used as input for two-phase partitioning. The upper fraction, for plasma membrane (PM), and the lower fraction for endomembranes (EM) were loaded at equal yield, followed by immunoblotting with α-myc and α-T7 to detect RPM1(*) and RIN4, respectively. Plasma membrane-localized (PM) ATPase and ER-localized BIP proteins represented the efficiency of fractioning for PM and EM.
(F) Conductivity measurements and HR phenotype after co-infiltration of RIN4 T166D with either RPM1 or an RPM1 G205E. N. benthamiana leaves were hand-infiltrated with Agrobacterium C58C1 strains expressing T166D mutant (OD600=0.4) and either pRPM1:RPM1:myc (OD600=0.4) or RPM1:myc G205E (OD600=0.8). C58C1 was used as filler to make up the difference in OD between RPM1:myc and RPM1:myc G205E with OD600=0.4. The measurements started two days post infiltration. This result was one of two repeats. Trypan Blue staining with leaf discs covering half of the infiltrated zone was performed 2.5 days after infiltration indicated 12 hr in conductivity measurement.
(G) Expression of RPM1:myc and RPM1:myc G205E. Protein samples from (F) were prepared 2 days post infiltration. The immunoblot was performed with α-myc.