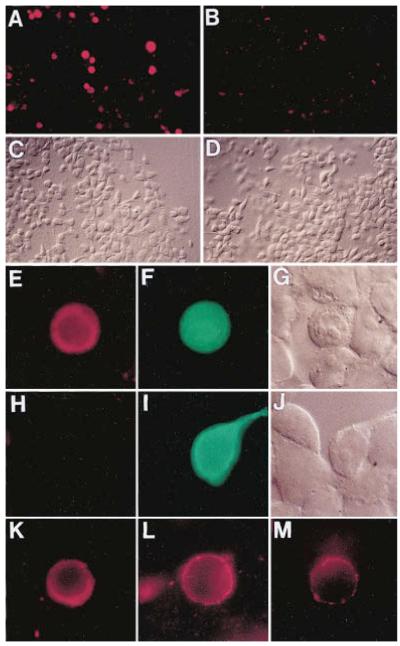
Figure 6.
Frzb-1 Protein Binds to Cells Expressing a Wnt-1/Transmembrane Chimera
(A) Low magnification view of 293 cells transfected with a Wnt1CD8 construct expressing Wnt-1 tethered to the membrane. After incubation in conditioned medium containing 250 nM Frzb1–HA protein and staining with anti-HA, positive cells appear as red spots.
(B) Cells transfected with the plasmid coding for truncated Chordin protein (pΔ-Chd), used here as a negative control.
(C) The same field of view as in (A) in Nomarski optics.
(D) Nomarski view of the same cells as in (B).
(E) High power view of a culture co-transfected with Wnt1CD8 and LacZ in pcDNA3.
(F) The same cell that binds Frzb1–HA (red in panel E) also expresses LacZ from the cotransfected plasmid (in green).
(G) Nomarski view of the same field as in (E) and (F).
(H) Cells cotransfected with the full-length CD8 control as well as LacZ plasmid and treated with the Frzb1–HA medium shows no specific staining.
(I) The same cells as in panel (H) are shown after staining for β-galactosidase (in green).
(J) The same field of cells as in (H) and (I) is shown using Nomarski optics.
(K) Binding of Frzb1–HA (250 nM) performed at 4°C for 2.5 hr on Wnt1CD8-transfected cells stained with anti-HA (in red).
(L) Incubation of cells expressing Wnt1CD8 chimera with Frzb1–HA diluted down to 125 pM.
(M) Cell transfected with a mutant Wnt1CD8 (cysteine 369 mutated to tryptophan) that reduces its biological activity is able to bind diluted Frzb1–HA (125 pM). This experiment was carried out simultaneously to the one illustrated in (L) but exposure time was four times longer. Stained cells in (K)–(L) were GFP positive (data not shown). All pictures were taken on a Zeiss Axiophot microscope with 40X (A–D) or 100X objectives (E–M). Photos were taken with a Kodak Ektachrome P1600 film and printed photographically.