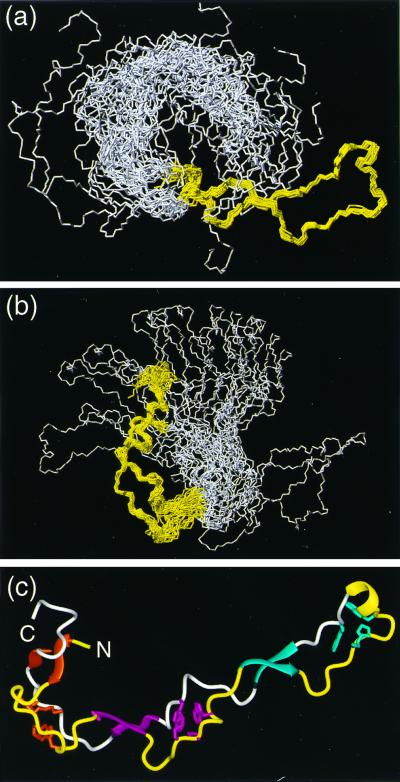
Figure 2.
(a and b) Bundles of the 20 energy-minimized conformers used to represent the NMR structure of CRT(189–288). (a) Superposition for best fit of the backbone atoms N, Cα, and C′ of the residues 219–258. (b) Superposition for best fit of the backbone atoms N, Cα, and C′ of the residues 189–209 and 262–284. In each drawing the polypeptide segments used for the superposition are colored yellow, and the remaining residues are white. (c) Cartoon of the conformer from a for which the white region is on the extreme left. The β-sheets and the helical turn on the extreme right are represented by ribbons and colored as in Fig. 1. The same color code is used for the three associated hydrophobic clusters. The polypeptide segments that connect the β-strands are drawn as thin cylindrical rods, which are yellow for the type 1 repeats and white for the type 2 repeats. Figs. 2–5 were prepared by using the program molmol (32).