Abstract
Using an in silico approach, we identified a putative zinc finger domain-containing transcription factor (zinc finger protein 105, ZFP105) that was enriched in the adult mouse testis. RT-PCR analyses showed that Zfp105 was indeed highly expressed in adult mouse testis and that its expression was regulated during postnatal development. To further characterize Zfp105 expression, we generated a Zfp105:β-galactosidase (LacZ) knock-in reporter mouse line (Zfp105LacZ/+) in which a Zfp105:LacZ fusion gene was expressed. Whole-mount LacZ analyses of adult Zfp105LacZ/+ tissues showed robust LacZ staining in the testis, very weak staining in the ovary and no staining in the spleen, liver, kidney, heart, lung, thymus, adrenal gland, uterus or oviduct. Sectional LacZ staining showed that ZFP105 was highly expressed in pachytene spermatocytes. ZNF35, the human ortholog of ZFP105, was also expressed in male germ cells of normal human testis. More importantly, reduced male fertility and sloughed spermatogenic cells were observed in adult Zfp105LacZ/LacZ mice. Taken together, our results suggest that ZFP105 is a male germ-cell factor and plays a role in male reproduction.
Keywords: knock-in, LacZ, ZNF35, spermatocyte, mouse
Introduction
Spermatogenesis is the process that occurs in the seminiferous tubules of the testis leading to the production of mature sperm (Setchell 1993). This process includes the spermatogonial phase, which involves the proliferation of cells via mitosis, the spermatocyte phase, in which recombination and meiosis occurs to yield haploid cells, and the spermatid stage when terminal differentiation of the haploid spermatids yields mature sperm (Setchell 1993). This complex process is at least partially controlled by transcriptional factors in the spermatocytes and round spermatids (Kimmins et al. 2004; Li et al. 2005; Maclean and Wilkinson 2005). Therefore, characterization of transcriptional factors in these male germ cells will provide insights into normal spermatogenesis and ultimately the pathogenesis of certain types of male infertility.
As part of our goal to characterize the transcriptional regulation network during spermatogenesis and male reproduction, we used an in silico-based approach (Lin and Matzuk 2005; Zhang et al. 2008) to identify candidate transcriptional factors. Using this strategy, we identified a testis-enriched putative transcriptional factor, zinc finger protein 105 (ZFP105). ZFP105 is a 58 kilodalton protein that contains 11 C2H2-type zinc finger domains located at the carboxyl (C)-terminus. These zinc finger domains are conserved among their orthologs in other vertebrates (www.ensembl.org). The human ortholog, ZNF35, can bind to the DNA sequence 5'-C/GC/GAAG/TA-3' and likely functions as a transcriptional activator (Lanfrancone et al. 1992; Pengue et al. 1993). It has been proposed that ZFP105 or ZNF35 will play a critical role in myeloid terminal differentiation, early embryonic development, spermatogenesis and/or tumorigenesis (Kohno et al. 1993; Lanfrancone et al. 1992; Okada et al. 2005; Pannuti et al. 1988; Przyborski et al. 1998; Yoshikawa et al. 2006). In this report, we analyzed Zfp105 mRNA expression in mouse tissues during postnatal development. More importantly we generated Zfp105:galactosidase (LacZ) reporter mice (Zfp105LacZ/+) and characterized the expression pattern of ZFP105 in adults. Expression of ZNF35, the human ortholog of Zfp105, in the testis was also characterized. Finally, fertility and histological analyses were performed on homozygous Zfp105:LacZ mice. Our results suggest that ZFP105 is a male germ cell factor and plays a role in normal male fertility.
Results
Dominant expression of Zfp105 in the mouse testis
To identify genes enriched in the mouse testis, we performed the cDNA Digital Gene Expression Displayer (DGED) analysis of mouse cDNA libraries (http://cgap.nci.nih.gov/Tissues/GXS), similar to the approach described previously (Lin and Matzuk 2005; Zhang et al. 2008). Expression of these genes was then validated using mouse microarray data generated from the Genomics Institute of the Novartis Research Foundation (http://symaltas.gnf.org) (Su et al. 2002). Using this strategy, we identified a testis-enriched gene, Zfp105. Multi-tissue mouse microarray data (http://symatlas.gnf.org/SymAtlas) show that Zfp105 is predominantly expressed in the testis (Su et al. 2002) (Figure 1A). To confirm the dominant expression of Zfp105 in the testis, we performed RT-PCR analyses with specific primers for Zfp105 and found the presence of abundant PCR products in adult mouse testes (Figure 1B). Weak expression of Zfp105 was also detected in the ovary (Figure 1B). However, there was no detectable Zfp105 expression in all other tested tissues (Figure 1B). These results are consistent with the above in silico prediction as well as a previous Northern blot analysis reported by Przyborski et al (Przyborski et al. 1998), indicating that Zfp105 is primarily expressed in the mouse testis at the mRNA level.
Figure 1.
Zfp105 expression in the mouse testis. (A) Microarray data from the Genomics Institute of the Novartis Research Foundation showing Zfp105 expression in adult mouse testes (Su et al. 2002). (B) Expression of Zfp105 in adult mouse testes by semi-quantitative RT-PCR analyses. Lanes: B, brain; K, kidney; T, testis; U, uterus; Sv, seminal vesicles; E, epididymis; Sp, spleen; Li, liver; O, ovary; Lu, lung; H, heart. PCR cycles: Zfp105, 26 cycles; β-actin, 22 cycles. (C) Testicular expression of Zfp105 during postnatal development by QRT-PCR analyses. Zfp105 mRNA levels were normalized by β-actin mRNA in each sample (mean ± standard errors, n=3).
Zfp105 expression in the mouse testis during postnatal development
To investigate Zfp105 expression during postnatal development, we performed quantitative RT-PCR (QRT-PCR) analyses of RNA isolated from mouse testes at day 5, 9, 12, 14, 21, 28 and 56 after birth. As shown in Figure 1C, low levels of Zfp105 mRNA were detected in testes of 5–12-day-old mice. However, high levels of Zfp105 mRNA were detected in testes of 14–56-day-old mice (Figure 1C). These results indicate that Zfp105 expression is developmentally regulated in the testis. Because pachytene spermatocytes are developed in 14-day-old or older testes but not in 12-day-old testes (Bellve 1993), our QRT-PCR studies (Figure 1C) indicate that Zfp105 is likely expressed in pachytene spermatocytes at the mRNA level.
Generation of Zfp105LacZ/+ reporter mice
To characterize Zfp105 expression at the protein level, we performed immunohistochemical studies with commercial antibodies against the human ortholog ZNF35 and our own antibodies generated against bacterially-expressed recombinant ZFP105. However, neither antibody detected specific signals in mouse tissues (data not shown). To circumvent the lack of specific ZFP105 antibodies, we generated a Zfp105:LacZ reporter mouse line using the Baygenomic gene-trap embryonic stem (ES) cell line TEA059. Gene trapping is a method of generating ES cells with insertional mutations (Stanford et al. 2001). Baygenomic gene trap ES cells are generated by insertion of a gene-trap vector which contains a splice-acceptor sequence upstream of a reporter gene, LacZ/Neomycin (Neo) (a fusion of LacZ and Neo) into introns, resulting in the creation of a fusion transcript containing sequence from exon(s) upstream to the insertion joined to the LacZ/Neo marker (Stryke et al. 2003). To validate the correct insertion of the gene-trap LacZ/Neo vector into the Zfp105 allele in the Baygenomic ES cell line TEA059, we performed genomic PCR analyses using primers P1 (recognizing DNA sequence upstream pf the insertion site of the gene-trap vector in Zfp105 allele) and P3 (recognizing the gene-trap vector) (Figure 2A). As shown in Figure 2B, a specific 500-bp PCR product was detected in Zfp105LacZ/+ but not wild type ES cells. These results demonstrate correct insertion of the reporter into the Zfp105 allele in the TEA059 ES cell line. To investigate whether the LacZ reporter can be expressed in the TEA059 ES cell line, X-gal assays were performed on cultured ES cells with or without treatment of all-trans-retinoic acid (RA). As shown in Figure 2C, there was weak LacZ staining in untreated Zfp105LacZ/+ ES cells. However, robust blue signals were observed in RA-treated Zfp105LacZ/+ ES cells. These results suggest that LacZ reporter gene can be expressed in differentiated TEA059 cells. To test whether LacZ reporter gene expression in RA-treated TEA059 cells reflects endogenous Zfp105 expression, we performed RT-PCR analyses to examine Zfp105 expression in wild type ES cells after RA treatment. As shown in Figure 2D, increased Zfp105 RNA levels were observed in RA-treated ES cells. Zfp105LacZ/+ ES cells (TEA059) were then injected into mouse blastocysts to generate chimeric mice. Breeding of chimeric males with wild type C57BL/6 females produced agouti pups. Genotype analyses of mouse tails from the agouti pups showed successful germline transmission of the Zfp105LacZ allele (Figure 2E). To determine whether the expression of LacZ is driven by the endogenous Zfp105 promoter in Zfp105LacZ/+ mice, we performed RT-PCR analysis of Zfp105LacZ/+ testes using a forward primer recognizing the exon 2 of Zfp105 and a reverse primer recognizing the LacZ gene (Figure 2F). As shown in Figure 2G, a 1.8-kb band was detected in Zfp105LacZ/+, but not in Zfp105+/+ testes. These results suggest that Zfp105:LacZ fusion gene is expressed in Zfp105LacZ/+ mice. Collectively, a Zfp105:LacZ reporter mouse line has been successfully generated.
Figure 2.
Generation of Zfp105LacZ/+ reporter mice. (A) Schematic representation of gene-trap Zfp105LacZ allele. P1, P2 and P3 are the primers for genotyping. (B) Genomic PCR analyses (using primers P1 and P3) showing the correct insertion of the gene trap vector in the Zfp105 allele. (C) Positive LacZ staining in Zfp105LacZ/+ ES cells following RA treatment for 48 hours. (D) RT-PCR showing RA-induced Zfp105 expression in wild type ES cells. The number of PCR cycles for Zfp105 and β-actin were 26 and 23, respectively. (E) PCR analyses showing germline transmission of the Zfp105LacZ allele. Tail DNA of four agouti pups produced from the breeding of a chimeric male with a C57BL/6 female were subjected to PCR analyses with the indicated PCR primers. (F) Schematic representation of the Zfp105:LacZ fusion transcript. RT-PCR primers are indicated by arrows. (G) RT-PCR analysis showing the expression of Zfp105:LacZ fusion RNA transcript in 2-month-old Zfp105LacZ/+ testes.
ZFP105 expression in adult Zfp105LacZ/+ mice
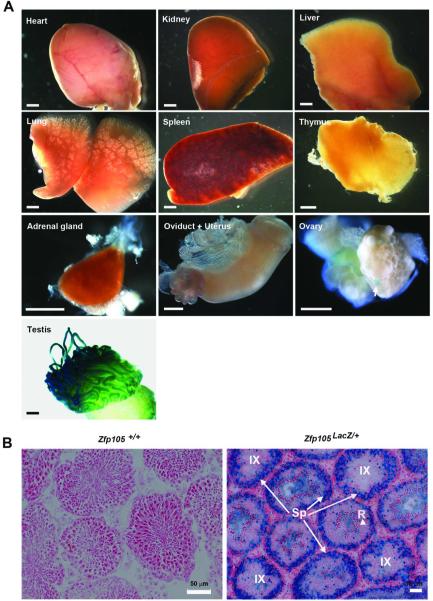
Having generated Zfp105LacZ/+ reporter mice, we performed whole-mount LacZ staining on various tissues from adult Zfp105+/+ and Zfp105LacZ/+ mice to determine ZFP105 expression (Figure 3A). As expected, there was no LacZ staining in tissues from control Zfp105+/+ mice (data not shown). In Zfp105LacZ/+ mice, strong positive staining was observed in the testis, but not in heart, kidney, liver, lung, spleen, thymus, adrenal gland, oviduct or uterus (Figure 3A). Weak LacZ staining was also observed in a few follicles of Zfp105LacZ/+ ovaries (Figure 3A). To further determine ZFP105 expression in the testis, we performed LacZ staining on frozen sections from Zfp105+/+ and Zfp105LacZ/+ mice (Figure 3B). As expected, there was no positive LacZ staining in Zfp105+/+ testis. In Zfp105LacZ/+ mice, there was very weak staining in interstitial cells, spermatogonia and round and elongating spermatids within the seminiferous tubules (Figure 3B). However, robust staining was observed in the spermatocytes (esp. pachytene spermatocytes) of adult Zfp105LacZ/+ testes (Figure 3B). These results suggest that ZFP105 is highly expressed in the testis with dominant expression in the spermatocytes.
Figure 3.
LacZ staining of adult tissues from Zfp105LacZ/+ mice. (A) Whole-mount LacZ staining of various tissues from adult Zfp105LacZ/+mice. Note the weak staining in some ovarian follicles (indicated by the white arrow) and the robust staining in the testis. Scale bar = 1 mm. (B) LacZ staining in the spermatocytes of adult Zfp105LacZ/+ (right panel) but not in Zfp105+/+ testes (left panel). Sp, spermatocytes (arrows); R, round spermatids (arrowhead). In the right panel, tubules at stage IX are indicated by IX, while the other tubules are likely at stages V–VI.
Expression of ZNF35, the Zfp105 human ortholog, in normal human testis
To investigate whether ZNF35 has an expression pattern similar to its mouse ortholog Zfp105, we first performed RT-PCR analyses using specific primers for ZNF35. As shown in Figure 4A, high levels of ZNF35 transcripts were detected in normal human testis, but not in other tested tissues including spleen, thymus, prostate, ovary, small intestine, colon and leukocytes. Then, immunofluorescence was performed using specific ZNF35 antibodies to examine ZNF35 expression in human testes. As shown in Figure 4B, no positive fluorescence signals were detected in the testis with normal rabbit IgG. However, positive fluorescence signals with anti-ZNF35 antibodies were detected in male germ cells within seminiferous tubules as well as in interstitial cells. Positive nuclear staining was detected in only a limited number of male germ cells (indicated by the arrow in Figure 4B), while the majority of male germ cells displayed cytosolic or perinuclear staining. The above results indicate that like Zfp105, ZNF35 is also highly expressed in the testis, particularly in the spermatogenic cells.
Figure 4.
ZNF35 expression in human testis. (A) RT-PCR showing high expression of ZNF35 mRNA in normal human testis. Sp, spleen; Th, thymus; P, prostate; T, testis; O, ovary; Si, small intestine; C, colon; Le, leukocytes. PCR cycles: ZNF35, 31 cycles; GAPDH, 26 cycles. (B) Immunofluorescent studies showing the presence of ZNF35 in spermatogenic cells within human testis. Nuclear staining is indicated by the arrow.
Reduced male fertility in Zfp105LacZ/LacZ mice
To test whether ZFP105 plays a role in male fertility, Zfp105LacZ/LacZ mice were generated and then subjected to fertility analysis. As shown in Figure 5A, homozygous LacZ reporter mice were successfully generated from the intercross of Zfp105LacZ/+ mice. There was no overt developmental defect in Zfp105LacZ/LacZ mice. Northern blot analysis using radiolabeled probes against exon 4 (encoding 11 C2H2 domains) showed the complete loss of RNA transcripts in Zfp105LacZ/LacZ testes (Figure 5B), indicating that the putative DNA binding domains of ZFP105 are absent in Zfp105LacZ/LacZ mice. To test if there is a fertility defect in Zfp105LacZ/LacZ mice, we performed fertility analysis of mutant mice and their wild type and heterozygous littermates for six-eight months. As shown in Figure 5C, no significant change in the number of litters per month was observed among tested Zfp105+/+, Zfp105LacZ/+, Zfp105LacZ/LacZ males or females during the breeding period. However, there was a significant decrease in the litter size produced from male Zfp105LacZ/LacZ mice, when compared to wild type and heterozygous males and homozygous female littermates (P < 0.05). These results suggest that loss of exon 4 from Zfp405 causes male subfertility.
Figure 5.
Fertility and histological analysis of Zfp105LacZ/LacZ mice. (A) Representative genotyping results of a litter of pups produced from heterozygous intercrosses. Pup #2, Zfp105+/+; Pup# 1, 3, 4 and 6, Zfp105LacZ/+; and Pup #5 and 7, Zfp105LacZ/LacZ. (B) Northern blot analysis showing the loss of full-length Zfp105 transcripts in Zfp105LacZ/LacZ testes using probes against the exon 4 of Zfp105. (C) Breeding results showing male subfertility in Zfp105LacZ/LacZ mice. Five to six mice of each genotype were bred with fertile males or females for a period of 6–8 months. Data are represented as mean ± standard error of the indicated number of samples. (D) Histological analysis of testes and epididymides of 3-month-old Zfp105LacZ/LacZ and control Zfp105LacZ/+ littermates. Note the presence of sloughed spermatogenic cells in the lumen of seminiferous tubules (indicated by arrowhead) and epididymis (indicated by arrows) from Zfp105LacZ/LacZ mice.
Sloughed spermatogenic cells in Zfp105LacZ/LacZ testes
To investigate whether there is a morphological defect in the testis, we performed histological analyses of testes and epididymides from Zfp105LacZ/LacZ and control Zfp105LacZ/+ littermates. All stages of seminiferous tubules during spermatogenesis were observed in Zfp105LacZ/LacZ and control Zfp105LacZ/+ littermates (data not shown). However, sloughed spermatogenic cells were present in the lumen of seminiferous tubules in Zfp105LacZ/LacZ testes but not in control littermates (Figure 5D, top two panels). Sloughed cells (likely the spermatogenic cells) in the epididymal lumen were also observed in Zfp105LacZ/LacZ but not Zfp105LacZ/+ littermates (Figure 5D, bottom two panels). These results suggest that Zfp405 plays a role in normal spermatogenesis.
Discussion
Using an in silico approach (Lin and Matzuk 2005; Zhang et al. 2008), we have identified a putative transcription factor ZFP105 enriched in the testis. Consistent with the in silico prediction and previous studies (Przyborski et al. 1998), Zfp105 is highly expressed in the testis (Figure 1B). Our QRT-PCR studies of Zfp105 mRNA in the testes during postnatal development (Figure 1C) suggest that Zfp105 is highly expressed in pachytene spermatocytes, given that pachytene spermatocyte is the latest stage of spermatogenic cells developed in the testis at 14 days after birth (Bellve 1993). Indeed, Zfp105 transcripts have been detected in pachytene spermatocytes by in situ hybridization and Northern blot analyses (Przyborski et al. 1998). More importantly, we have successfully generated Zfp105:LacZ reporter mice in which the Zfp105:LacZ fusion gene is expressed (Figure 2G). LacZ expression in adult Zfp105LacZ/+ tissues (Figure 3) and RA-treated gene-trap Zfp105 ES cells (Figure 2C) are consistent with Zfp105 mRNA expression in adult mouse tissues (Figure 1B) (Przyborski et al. 1998) and wild type ES cells (Figure 2D), respectively. Induced Zfp105 expression in RA-treated ES cells (Figure 2C–D) is also consistent with the original identification of Zfp105 (Przyborski et al. 1998) using a probe against a zinc finger transcript upregulated in RA-treated human embryonal carcinoma NTERA2 cells (Andrews 1984). Robust LacZ expression in pachytene spermatocytes of adult Zfp105:LacZ reporter mice correlates well with Zfp105 mRNA expression in those cells (Przyborski et al. 1998), even though our LacZ expression results in postmeoitic spermatids of Zfp105LacZ/+ testes are different from Zfp105 mRNA expression as reported previously (Przyborski et al. 1998). Together, our results suggest that Zfp105 is highly expressed in the testis, especially in pachytene spermatocytes. Like Zfp105, ZNF35 was also highly expressed in human testis and ZNF35 protein was detected in spermatogenic cells (Figure 4A–B). Collectively, we have successfully characterized the germ cell-expression of Zfp105 and its human ortholog in adult testes.
Male germ-cell expression of Zfp105 and ZNF35 characterized here suggests that they may play a critical role in spermatogenesis and affect male fertility. Indeed, we have observed reduced fertility in homozygous Zfp105:LacZ reporter male mice (Figure 5C). Reduced male fertility is likely caused by sloughing of immature spermatogenic cells into the testicular lumen in Zfp105LacZ/LacZ mice (Figure 5D). Our results suggest that ZFP105 plays a role in normal spermatogenesis and ultimately male fertility. However, the underlying molecular mechanism of ZFP105 action during spermatogenesis and male fertility remains to be elucidated. One potential mechanism of ZFP105 action in spermatogenic cells, however, is to bind to the putative ZFP105/ZNF35 binding sites (5'-C/GC/GAAG/TA-3') (Pengue et al. 1993) and control the transcription of male germ-cell-specific genes. Since Zfp105 expression can be induced by RA in ES cells (Figure 2C–D), it is possible that a similar scenario occurs in the adult testis. ZFP105 could be a target of RA signaling that are important for normal spermatogenesis (Chung et al. 2004; Dufour and Kim 1999; Kastner et al. 1996). In addition, ZFP105 may function as an RNA binding protein like other zinc finger proteins such as REX-1/ZFP42 (Rogers et al. 1991), KIN 17 (Pinon-Lataillade et al. 2004) and ZFR (Meagher et al. 1999) to elicit its biological function in spermatogenic cells (e.g. spermatocytes). Regardless, our studies on Zfp105LacZ/LacZ mice provide direct evidence about the role of ZFP105 in male fertility.
It should be emphasized here that Zfp105LacZ/LacZ mice unlikely are Zfp105 null mutants. This is indicated by the presence of Zfp105:LacZ fusion transcripts in Zfp105LacZ/+ testes (Figure 2F–G). Although full-length Zfp105 RNA transcripts were not detected in Zfp105LacZ/LacZ testes by radiolabeled probes against the C2H2 domain-coding cDNA (exon 4) (Figure 5B), the first 110 amino-acid residues of ZFP105 (encoded by the exons 2 and 3 of Zfp105) could be translated from the Zfp105:LacZ fusion transcripts and have a role in male fertility. This may explain why there is a relatively minor reduction in male fertility displayed in Zfp105LacZ/LacZ mice. In addition, there exists a ZNF35 paralog, ZNF660 that contains 10 C2H2 domains, in human genome (www.ensembl.org). The potential existence of the Zfp105 paralog, Zfp660, could compensate the loss of the C-terminus of ZFP105 in Zfp105LacZ/LacZ mice and mask the male fertility phenotypes. Furthermore, many other C2H2-type zinc finger proteins such as ZFR (containing 3 C2H2 repeats), ZFP42 (containing 4 C2H2 repeats) and KIN17 (containing 11 C2H2 domain) have been shown to be expressed in mouse spermatocytes (Meagher et al. 1999; Pinon-Lataillade et al. 2004; Rogers et al. 1991). These zinc finger proteins may have some functional redundancies with ZFP105 in spermatocytes to regulate spermatogenesis and male fertility. Nevertheless, our studies suggest that the C-terminus of ZFP105 (containing 11 C2H2 domains encoded by single exon 4) plays a role in normal spermatogenesis and male fertility.
It has been postulated that Zfp105 may be involved in early embryonic development and tumorigenesis (Kohno et al. 1993; Lanfrancone et al. 1992; Okada et al. 2005; Pannuti et al. 1988; Przyborski et al. 1998; Yoshikawa et al. 2006). However, embryonic lethality was not observed in Zfp105LacZ/LacZ mice. There were no gross defects in Zfp105LacZ/LacZ mice during postnatal development. We also did not observe any tumor in the testis or other major organs from Zfp105LacZ/LacZ mice up to 12 months of age (data not shown). Therefore, our studies indicate that the C-terminal ZFP105 encoded by the exon 4 is not important for normal embryonic development and tumorigenesis, except the aforementioned male fertility.
In conclusion, we have successfully identified and characterized Zfp105 expression in adult mice. Our results suggest that ZFP105 is a male-germ cell factor in adults. More importantly, our studies provide direct evidence on the role of Zfp105 in male fertility.
Materials and methods
In silico analysis
Mouse cDNA sequences enriched in the testis were obtained using the cDNA Digital Gene Expression Displayer (DGED) program (http://cgap.nci.nih.gov/Tissues/GXS), similar to previous reports (Lin and Matzuk 2005; Zhang et al. 2008) . Expression of these genes in the testis was then examined on multi-tissue mouse microarray data generated from the Genomics Institute of the Novartis Research Foundation (http://symaltas.gnf.org) (Su et al. 2002).
ES cell culture
Gene-trap Zfp105 ES cells (TEA059) were purchased from BayGenomics (University of California at Davis, California). These cells were cultured in ES cell media containing 400 μg/ml G418 (Invitrogen, Carlsbad, CA) on irradiated SNL feeder cells expressing murine leukemia inhibitory factor, as described previously (Mudgett and Livelli 1995). Wide type (AB1.2) and gene-trap Zfp105LacZ/+ ES cells were incubated with 10−6 M RA (Sigma Chemical Co., St. Louis, MO) to induce differentiation.
Animals, genotyping and tissue collection
Adult C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, Maine) and maintained on a 14 hour light:10 hour dark cycle, with free access to food and water, in the vivarium of the University of Louisville. Zfp105LacZ/+ ES cells were injected into C57BL/6 blastocysts to generate male chimeras with 50–100% agouti color. These chimeric mice were bred with female C57BL/6 mice to generate Zfp105LacZ/+ mice. Zfp105LacZ/+ mice were then intercrossed to obtain Zfp105LacZ/LacZ mice. Genomic DNA of Zfp105LacZ/+ ES cells and mouse tails were extracted and genotyped by PCR (Lan et al. 2003c) using primers 5'-atgtgtttgcatgggtcaga-3' (P1), 5'-aaaggggaagggcaaaacta-3' (P2) and 5'-aaaggggaagggcaaaacta-3' (P3). Animals were euthanized with carbon dioxide and tissues were collected for RNA isolation, LacZ staining or fixation. Frozen sections were prepared as described previously (Zhang et al. 2008). The stage of seminiferous tubules within the testis was determined as described previously (Russell et al. 1990). All animal studies were conducted according to ethical guidelines and approved by the Animal Welfare and User Committee of University of Louisville.
RNA isolation, RT-PCR and Northern blot analysis
Total RNA from mouse tissues and RA-treated ES cells was isolated with Trizol reagent (Invitrogen, Carlsbad, CA). Semi-quantitative RT-PCR analyses with specific primers for Zfp105, Zfp105:LacZ fusion gene and β-actin were performed as described previously (Lan et al. 2003b). PCR analyses of ZNF35, and glyceraldedyde-3-phosphate dehydrogenase (GAPDH) were performed on normal human tissue cDNA (Catalog# K1421–1, Clontech Laboratories, Inc., ZNF35, and glyceraldedyde-3-phosphate dehydrogenase (GAPDH) Mountain View, CA). PCR primer sequences are as follows: Zfp105+/+ , 5'-taagtcaggcatccagccaac-3' (105-F) and 5'-ctcccaaatctcccttttcctc-3' (105-R), 438-bp products; Zfp105:LacZ fusion gene, 5'-taagtcaggcatccagccaac-3' (105-F) and 5'-atacagcgcgtcgtgattag-3'(LacZ-R), 1.8-kb products; ZNF35, 5'-gcggtagtcctgaaagcaac-3' and 5'-caacgaggtttgcactctga-3', 499-bp products; GAPDH, 5'-tgaaggtcggagtcaacggatttggt-3' and 5'-catgtagggccatgaggtccaccac-3', 983-bp products; and β-actin, 5'-ttgagaccttcaacacccc-3' and 5'-agccagagcagtaatctcc-3', 593-bp products. QRT-PCR analyses were performed as described previously (Lan et al. 2003b). QRT-PCR primers and Taqman® probes for Zfp105 are 5'-atggaagcatcagtgtatcagaagat-3', 5'-gcttttagcaccactgccatt-3', and 5'-FAM-aaagggtcaggaaatgttccggg-TAMRA-3' (Biosource International, Inc. Camarillo, CA). Northern blot analysis was performed using random-labeled probes against the exon 4 of Zfp105 or β-actin, as described previously (Lan et al. 1999).
LacZ staining and immunofluorescent studies
LacZ staining of RA-induced ES cells or tissue samples was performed according to the manufacturers' protocol (Specialty Media, Phillipsburg, NJ). Immunofluorescent studies were performed on paraffin-embedded normal human testis sections (purchased from US Biomax, Inc, Rockville, MD) using anti-ZNF35 antibodies (GenWay Biotech, Inc., San Diego, CA), as described previously (Lan et al. 2003a).
Male fertility studies
Five to six Zfp105+/+, Zfp105LacZ/+ and Zfp105LacZ/LacZ littermates were bred with proven fertile C57BL/6 females or males (1 male/1 female per cage) for 6–8 months. Litter sizes and litters per month were recorded as described previously (Lan et al. 2003b). Student's t-test was performed to determine the significant difference in litter size and the number of litters per month among each group.
Histological analysis
For histological analysis, tissues were fixed in 4% paraformaldehyde, dehydrated and embedded in paraffin. Paraffin-embedded tissues were sectioned at 7-μm thickness using a Richard-Allan Scientific MICROM HM325 microtome (Fisher Scientific, Pittsburgh, PA), and then stained with hematoxylin and eosin (H & E). Stained sections were examined under an Axio-Imager A1 microscope (Carl Zeiss Inc, Gottingen, Germany). Sections from at least three animals of each genotype were analyzed.
Acknowledgments
We thank Drs. Austin J. Cooney and Francesco J. DeMayo (Baylor College of Medicine) for injection of gene-trap Zfp105 ES cells. We also thank Dr. Dennis R. Warner for critical comments of this manuscript. This study was supported by NIH NCRR COBRE Grant#2P20-RR/DE17702-06 (to ZJL) and in part by NIH grant #5R01 HD57501-01 (to Zhenmin Lei).
References
- Andrews PW. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev Biol. 1984;103:285–293. doi: 10.1016/0012-1606(84)90316-6. [DOI] [PubMed] [Google Scholar]
- Bellve AR. Purification, culture, and fractionation of spermatogenic cells. Methods Enzymol. 1993;225:84–113. doi: 10.1016/0076-6879(93)25009-q. [DOI] [PubMed] [Google Scholar]
- Chung SS, Sung W, Wang X, Wolgemuth DJ. Retinoic acid receptor alpha is required for synchronization of spermatogenic cycles and its absence results in progressive breakdown of the spermatogenic process. Dev Dyn. 2004;230:754–766. doi: 10.1002/dvdy.20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour JM, Kim KH. Cellular and subcellular localization of six retinoid receptors in rat testis during postnatal development: identification of potential heterodimeric receptors. Biol Reprod. 1999;61:1300–1308. doi: 10.1095/biolreprod61.5.1300. [DOI] [PubMed] [Google Scholar]
- Kastner P, Mark M, Leid M, Gansmuller A, Chin W, Grondona JM, Decimo D, Krezel W, Dierich A, Chambon P. Abnormal spermatogenesis in RXRβ mutant mice. Genes Dev. 1996;10:880–892. doi: 10.1101/gad.10.1.80. [DOI] [PubMed] [Google Scholar]
- Kimmins S, Kotaja N, Fienga G, Kolthur US, Brancorsini S, Hogeveen K, Monaco L, Sassone-Corsi P. A specific programme of gene transcription in male germ cells. Reprod Biomed Online. 2004;8:496–500. doi: 10.1016/s1472-6483(10)61094-2. [DOI] [PubMed] [Google Scholar]
- Kohno T, Takayama H, Hamaguchi M, Takano H, Yamaguchi N, Tsuda H, Hirohashi S, Vissing H, Shimizu M, Oshimura M, et al. Deletion mapping of chromosome 3p in human uterine cervical cancer. Oncogene. 1993;8:1825–1832. [PubMed] [Google Scholar]
- Lan ZJ, Gu P, Xu X, Cooney AJ. Expression of the orphan nuclear receptor, germ cell nuclear factor, in mouse gonads and preimplantation embryos. Biol Reprod. 2003a;68:282–289. doi: 10.1095/biolreprod.102.008151. [DOI] [PubMed] [Google Scholar]
- Lan ZJ, Gu P, Xu X, Jackson KJ, DeMayo FJ, O'Malley BW, Cooney AJ. GCNF-dependent repression of BMP-15 and GDF-9 mediates gamete regulation of female fertility. EMBO J. 2003b;22:4070–4081. doi: 10.1093/emboj/cdg405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan ZJ, Lye RJ, Holic N, Labus JC, Hinton BT. Involvement of polyomavirus enhancer activator 3 in the regulation of expression of gamma-glutamyl transpeptidase messenger ribonucleic acid-IV in the rat epididymis. Biol Reprod. 1999;60:664–673. doi: 10.1095/biolreprod60.3.664. [DOI] [PubMed] [Google Scholar]
- Lan ZJ, Xu X, Cooney AJ. Generation of a germ cell nuclear factor conditional allele in mice. Genesis. 2003c;37:172–179. doi: 10.1002/gene.10239. [DOI] [PubMed] [Google Scholar]
- Lanfrancone L, Pengue G, Pandolfi PP, Salcini AE, Giacomucci A, Longo L, Donti E, De Luca P, La Mantia G, Pelicci PG, et al. Structural and functional organization of the HF.10 human zinc finger gene (ZNF35) located on chromosome 3p21–p22. Genomics. 1992;12:720–728. doi: 10.1016/0888-7543(92)90301-8. [DOI] [PubMed] [Google Scholar]
- Li B, Nair M, Mackay DR, Bilanchone V, Hu M, Fallahi M, Song H, Dai Q, Cohen PE, Dai X. Ovol1 regulates meiotic pachytene progression during spermatogenesis by repressing Id2 expression. Development. 2005;132:1463–1473. doi: 10.1242/dev.01658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YN, Matzuk MM. High-throughput discovery of germ-cell-specific genes. Semin Reprod Med. 2005;23:201–212. doi: 10.1055/s-2005-872448. [DOI] [PubMed] [Google Scholar]
- Maclean JA, 2nd, Wilkinson MF. Gene regulation in spermatogenesis. Curr Top Dev Biol. 2005;71:131–197. doi: 10.1016/S0070-2153(05)71005-X. [DOI] [PubMed] [Google Scholar]
- Meagher MJ, Schumacher JM, Lee K, Holdcraft RW, Edelhoff S, Disteche C, Braun RE. Identification of ZFR, an ancient and highly conserved murine chromosome-associated zinc finger protein. Gene. 1999;228:197–211. doi: 10.1016/s0378-1119(98)00615-5. [DOI] [PubMed] [Google Scholar]
- Mudgett JS, Livelli TJ. Electroporation of embryonic stem cells for generating transgenic mice and studying in vitro differentiation. Methods Mol Biol. 1995;48:167–184. doi: 10.1385/0-89603-304-X:167. [DOI] [PubMed] [Google Scholar]
- Okada A, Kushima K, Aoki Y, Bialer M, Fujiwara M. Identification of early-responsive genes correlated to valproic acid-induced neural tube defects in mice. Birth Defects Res A Clin Mol Teratol. 2005;73:229–238. doi: 10.1002/bdra.20131. [DOI] [PubMed] [Google Scholar]
- Pannuti A, Lanfrancone L, Pascucci A, Pelicci PG, La Mantia G, Lania L. Isolation of cDNAs encoding finger proteins and measurement of the corresponding mRNA levels during myeloid terminal differentiation. Nucleic Acids Res. 1988;16:4227–4237. doi: 10.1093/nar/16.10.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pengue G, Cannada-Bartoli P, Lania L. The ZNF35 human zinc finger gene encodes a sequence-specific DNA-binding protein. FEBS Lett. 1993;321:233–236. doi: 10.1016/0014-5793(93)80115-b. [DOI] [PubMed] [Google Scholar]
- Pinon-Lataillade G, Masson C, Bernardino-Sgherri J, Henriot V, Mauffrey P, Frobert Y, Araneda S, Angulo JF. KIN17 encodes an RNA-binding protein and is expressed during mouse spermatogenesis. J Cell Sci. 2004;117:3691–3702. doi: 10.1242/jcs.01226. [DOI] [PubMed] [Google Scholar]
- Przyborski SA, Knowles BB, Handel MA, Gurwitch SA, Ackerman SL. Differential expression of the zinc finger gene Zfp105 during spermatogenesis. Mamm Genome. 1998;9:758–762. doi: 10.1007/s003359900859. [DOI] [PubMed] [Google Scholar]
- Rogers MB, Hosler BA, Gudas LJ. Specific expression of a retinoic acid-regulated, zinc-finger gene, Rex-1, in preimplantation embryos, trophoblast and spermatocytes. Development. 1991;113:815–824. doi: 10.1242/dev.113.3.815. [DOI] [PubMed] [Google Scholar]
- Russell LD, Ettlin RA, Hikim APS, Clegg ED. Histological and histopathological evaluation of the testis. Cache River Press; Clearwater, FL: 1990. pp. 120–161. [Google Scholar]
- Setchell BP. Spermatogenesis and Spermatozoa. In: Austin CR, Short RV, editors. Reproduction in Mammals: Germ Cells and Fertilization. 2nd edition ed Cambridge University Press; 1993. pp. 63–101. [Google Scholar]
- Stanford WL, Cohn JB, Cordes SP. Gene-trap mutagenesis: past, present and beyond. Nat Rev Genet. 2001;2:756–768. doi: 10.1038/35093548. [DOI] [PubMed] [Google Scholar]
- Stryke D, Kawamoto M, Huang CC, Johns SJ, King LA, Harper CA, Meng EC, Lee RE, Yee A, L'Italien L, Chuang PT, Young SG, Skarnes WC, Babbitt PC, Ferrin TE. BayGenomics: a resource of insertional mutations in mouse embryonic stem cells. Nucleic Acids Res. 2003;31:278–281. doi: 10.1093/nar/gkg064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su AI, Cooke MP, Ching KA, Hakak Y, Walker JR, Wiltshire T, Orth AP, Vega RG, Sapinoso LM, Moqrich A, Patapoutian A, Hampton GM, Schultz PG, Hogenesch JB. Large-scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci U S A. 2002;99:4465–4470. doi: 10.1073/pnas.012025199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T, Piao Y, Zhong J, Matoba R, Carter MG, Wang Y, Goldberg I, Ko MS. High-throughput screen for genes predominantly expressed in the ICM of mouse blastocysts by whole mount in situ hybridization. Gene Expr Patterns. 2006;6:213–224. doi: 10.1016/j.modgep.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Denhard LA, Zhou H, Liu LH, Lan ZJ. 0610009K11Rik, a testis-specific and germ cell nuclear receptor-interacting protein. Biochem Biophys Res Commun. 2008;366:898–904. doi: 10.1016/j.bbrc.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]