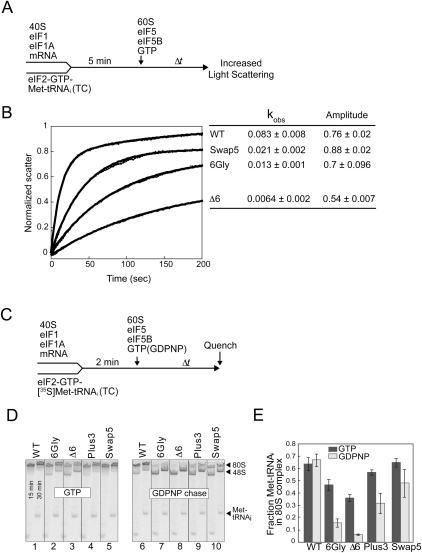
FIGURE 4.
Helix H12 mutants of eIF5B impair 80S complex stability. (A) Experimental scheme for subunit joining monitored by light scattering. (B) Subunit joining assay for WT eIF5B or the Swap5, 6Gly, or D6 mutant in the presence of GTP. Observed rate constant (kobs) and amplitude for each reaction are shown at the right end of the corresponding curve. Data are means ± standard error from at least two independent experiments. (C) Experimental scheme for 80S complex formation assay. (D,E) Results from 80S complex formation assay. 48S complexes were formed using limiting amounts of eIF2 ternary complexes containing [35S]Met-tRNAiMet and then subunit joining was monitored in the presence of either GTP (left panel) or GDPNP (right panel). Reactions were stopped at 15 and 30 min and loaded onto a running native gel. Following phosphorimage analysis of the gel (D), the amounts of [35S]Met-tRNAiMet that were free or bound to 43S or 80S complexes at 30 min were quantified, and the fraction of [35S]Met-tRNAiMet in 80S complexes was calculated (E). Data represent the mean of three independent experiments ± standard error.