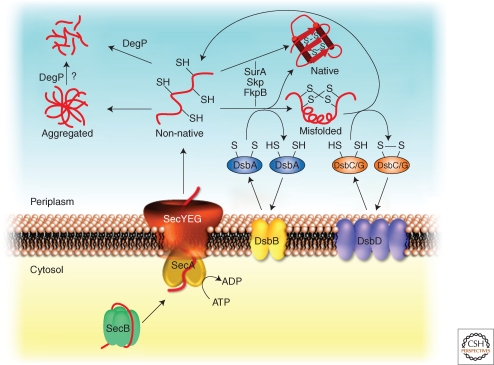
Figure 5.
Periplasmic quality control system. In the cytosol secretory proteins are kept in an export-competent conformation through association with the chaperone SecB. On delivery to SecA, the secretory proteins are translocated through the SecYEG translocon into the periplasm. Folding of newly imported proteins is supported by periplasmic chaperones including Skp, FkpB or SurA. Oxidative folding (formation of disulfide bonds) is catalyzed by DsbA, whereas incorrect disulfides are isomerized by DsbC/DsbG. Oxidized DsbA and DsbC/G are regenerated by DsbB and DsbD, respectively. Misfolded proteins are degraded by DegP. High levels of misfolded protein species can cause the generation of protein aggregates that might be removed by DegP.