Abstract
Placental tissue expresses many lymphatic markers. The current study was undertaken to examine if D2-40/podoplanin, a lymphatic endothelial marker, was expressed in the human placentas, and how it is altered developmentally and pathologically. We studied D2-40/podoplanin and VEGFR-3 expressions in placentas from normotensive pregnancies at different gestational ages and in placentas from women with clinically defined preeclampsia. D2-40 expression in systemic lymphatic vessel endothelium served as a positive control. Protein expression for D2-40, VEGFR-3, and β-actin were determined by Western blot in placentas from normotensive (n=6) and preeclamptic (n=5) pregnancies. Our results show that D2-40/podoplanin was strongly expressed in the placenta, mainly as a network plexus pattern in the villous stroma throughout gestation. CD31 was limited to villous core fetal vessel endothelium and VEGFR-3 was found in both villous core fetal vessel endothelium and trophoblasts. D2-40/podoplanin expression was significantly decreased, and VEGFR-3 significantly increased in preeclamptic placental tissues compared to normotensive placental controls. Placental villous stroma is a reticular-like structure, and the localization of D2-40 to the stroma suggests that a lymphatic-like conductive network may exist in the human placenta. D2-40/podoplanin is an O-linked sialoglycoprotein. Although little is known regarding biological functions of sialylated glycoproteins within the placenta, placental D2-40/podoplanin may support fetal vessel angiogenesis during placenta development and reduced D2-40/podoplanin expression in preeclamptic placenta may contribute to altered interstitial fluid homeostasis and impaired angiogenesis in this pregnancy disorder.
Keywords: D2-40/podoplanin, placenta, preeclampsia
Introduction
During pregnancy, maternal uterine blood vessels undergo dramatic vascular remodeling to accommodate the increased utero-placental blood flow, which supports growth and development of the placenta and the fetus. Adequate utero-placental blood flow is essential for normal placental perfusion and critical to fetal health, survival and successful pregnancy. Recent work indicates that like uterine blood vessels, dramatic changes in the uterine lymphatic vessel system also take place in the vascular remodeling process during pregnancy1, 2.
In general, the lymphatic vasculature acts for a parallel collection circuit to blood vessels in most tissues and organs, possibly even the brain3, actively regulating fluid balance, lipid transport and immune cell trafficking. Lymphatics are the main route for collection of filtered interstitial fluid and cells in tissues. Although the lymphatic system plays an important role in vessel remodeling and fluid homeostasis in the uterus during pregnancy, no lymphatic vessels have been reported in the placenta and interstitial fluid homeostasis in the placenta remains enigmatic. Recently, a study by our group did show that many lymphatic-associated markers are expressed in the placenta including VEGF-C, VEGF-D, and VEGFR-3/Flt-44. Lymphatic vascular hyaluronan receptor LYVE-1 is expressed in trophoblasts, which appears to compensate for the absence of CD44 in the placenta4.
D2-40 is a recently developed monoclonal antibody raised against an M2A antigen5, 6, a Mr 40,000 surface sialoglycoprotein associated with germ cell neoplasia and fetal testicular gonocytes. In tumor pathology, D2-40 has been used to detect lymphatic invasion in tumor tissues. D2-40 specifically recognizes human podoplanin, and has been demonstrated as a selective marker for lymphatic endothelium. To determine if D2-40/podoplanin is expressed in the human placenta, immunoreactivity of D2-40/podoplanin was examined in the 1st, 2nd, and 3rd trimester placentas. VEGFR-3, the main lymphatic endothelial growth factor receptor, and CD31, a marker for blood vessel endothelium, were also examined. There was a unique pattern of D2-40 expression in villous stroma in the human placenta. D2-40/podoplanin was expressed in the human placenta throughout pregnancy and its expression was significantly reduced in placentas from preeclamptic pregnancies. This suggests that D2-40/podoplanin is developmentally expressed in the normally human placenta, and that alterations in D2-40 expression during preeclampsia may underlie interstitial imbalances seen in this pregnancy disorder.
Materials and Methods
Tissue sample collection
The third trimester placentas from normotensive and preeclamptic pregnancies were collected from the main hospital, Louisiana State University Health Sciences Center in Shreveport (LSUHSC-S). First and second trimester placentas were collected from selective pregnancy terminations at the Department of Obstetrics and Gynecology, the First Hospital of Harbin Medical University, China. Placenta collection was approved from both Institutions. Normal pregnancy is defined as maternal blood pressure lower than 140/90mm Hg with no other medical complications. Preeclampsia is defined as blood pressure higher than 140/90mm Hg for at least two separate readings with proteinuria (>1+) at dipstick, or higher than 300mg protein/24h urine. Placentas from patients showing signs of infection were excluded from this study, as were smokers.
Subcutaneous fat tissue from normotensive pregnant women was obtained during cesarean section delivery. Subcutaneous fat tissue sections were stained and used as control for D2-40 expression of lymphatic vessel endothelium. Tissue collection was approved by the IRB at LSUHSC-S.
Immunohistochemsitry
Expressions of D2-40/podoplanin and CD31 were examined by immunohistochemistry in tissue sections of placentas from 1st, 2nd, and 3rd trimester pregnancies. D2-40/podoplanin and VEGFR-3 expressions were also examined in placental tissues delivered by normotensive and preeclamptic pregnant women. The immunohistochemistry procedure was performed as previously described7. Briefly, tissue sections were deparaffinized with xylene and run through graded ethanol series. Antigen retrieval was accomplished by boiling tissue slides in citrate buffer, and endogenous peroxidase was quenched by 0.1% hydrogen peroxide (30mins). After blocking with goat serum, the slides were incubated with mAb of D2-40 (Covance), CD31 (Dako), VEGFR-3 (Santa Cruz), and anti-human podoplnin antibody (Abcam) at 4C over night. Slides stained with the same antibody were processed all together for uniformity. Corresponding secondary antibodies, AB enzyme and DAB detection kit were purchased from Vector Laboratories, Inc. (Burlingame, CA). The nuclei were counterstained with haematoxylin. Stained slides were examined by an Olympus microscope (Olympus IX71, Tokyo, Japan). Images were captured by a digital camera with PictureFrame computer software (Uptronics, Inc., Sunnyvale, CA) and recorded via microscope-linked PC.
Protein expression
Expressions of D2-40/podoplanin and VEGFR-3 were also examined by Western blotting using tissue homogenate samples. An aliquot of 50μg total protein was used to run SDS-PAGE and transferred to Hybond-protein transfer membrane (Amersham Corp, Arlington Heights, Ill). The membrane was probed with antibodies against D2-40/podoplanin and VEGFR-3. Bands were visualized with an enhanced chemiluminescent detection kit (Amersham). β-actin expression was also determined. Band density was scanned and analyzed by NIH Image 1.16. Relative densities for D2-40/podoplanin and VEGFR-3 expressions were normalized using βactin expression in each sample.
Statistical Analysis
The protein expression data for D2-40/podoplanin and VEGFR-3 between normotensive and preeclamptic placentas are expressed as mean ± SE. Statistical analysis was performed using unpaired t-test and Whitney U-test by computer software Statview (Cary, NC). A p<0.05 was considered statistically significant.
Results
D2-40/podoplanin expression in villous stroma
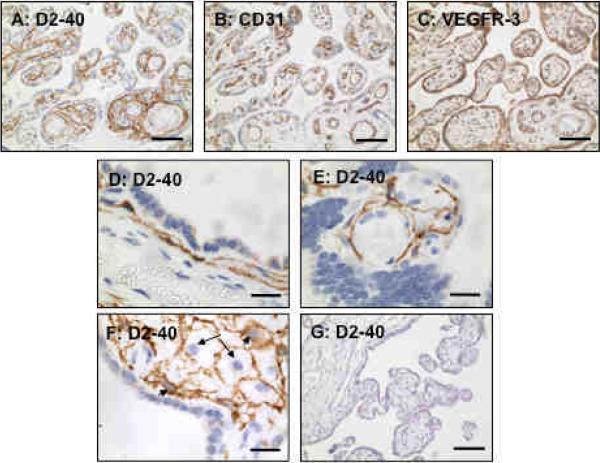
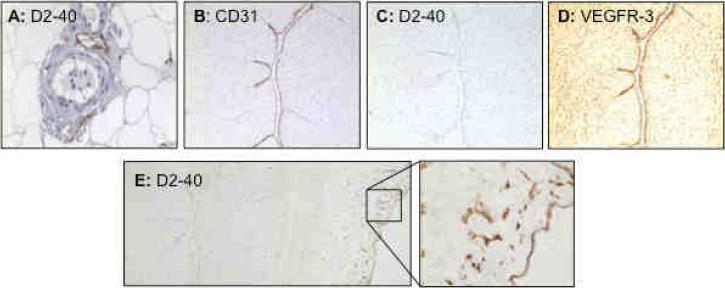
To determine if D2-40/podoplanin is expressed in the human placenta, we first examined immunoreactivities of D2-40, CD31, and VEGFR-3 by immunostaining of serial tissue sections of placentas from normotensive pregnancies (n=3). CD31 was used as a blood vessel endothelial marker and VEGFR-3 was used as a lymphatic vessel endothelial marker. Very interestingly, a strong D2-40 expression was detected in the villous stroma (Figure 1A), in the area of sub-syncytiotrophoblasts towards stroma (Figure 1D), and around villous core fetal vessels (Figure 1E). Positive D2-40 staining was also noticed in some stromal cells (Figure 1F). In contrast, CD31 was only expressed in the villous core fetal vessel endothelium (Figure 1B) while VEGFR-3 was expressed in both villous core fetal vessel endothelium and syncytiotrophoblasts (Figure 1C). Tissue sections stained with isotype IgG (Figure 1G) or only stained with secondary antibody showed negative staining. To further determine positive D2-40 expression in lymphatic vessels, but not in blood vessels, we examined D2-40 expression in systemic subcutaneous fat tissue sections and umbilical cord sections. Our results confirmed that D2-40 was expressed only in lymphatic vessel endothelium and not in blood vessel endothelium (Figure 2A). Umbilical vein endothelium expresses both CD31 and VEGFR-3, but not D2-40, further confirming D2-40 expression in the lymphatic tissue (Figure 2) (n=3). However, D2-40 was detected in the amniotic epithelium and underlying Wharton's Jelly matrix (Figure 2E). These results suggest that D2-40 reacts with podoplanin or podoplanin-like polypeptides localized in the villous stroma within the umbilical cord and placenta.
Figure 1.
Immunostaining of D2-40, CD31, and VEGFR-3 in serial villous tissue sections of a normal term placenta. Please note the compartmental difference in D2-40 (A, in stroma), CD31 (B, in villous core vessel endothelium), and VEGFR-3 (C, both trophoblasts and villous core vessel endothelium) distributions in placental villous tissue. Bar = 100 micron for A, B, and C. High magnification for D2-40 staining is shown in D (in the area of sub-syncytiotrophoblasts), E (around villous core fetal vessels) and F (some stromal cells, arrow: negative staining cells and arrowhead: positive staining cells), bar = 20 micron. Isotype IgG negative staining is shown in F, bar = 100 micron.
Figure 2.
Immunostaining of D2-40 in systemic vessels, and D2-40, CD31, and VEGFR-3 in placental umbilical cord. A: D2-40 staining in maternal vessels from normal pregnant women. Positive D2-40 staining is only seen in lymphatic vessel endothelium, but not in arterial or venous blood vessel endothelium. B, C, and D: Immunostaining of CD31, D2-40, and VEGFR-3 in umbilical cord, respectively. Negative D2-40 (C), but positive CD31 (B) and VEGFR-3 (C), staining is seen in umbilical vein endothelium. E: Positive D2-40 staining is also observed in cord membrane (amniotic epithelium) and underlying matrix (Wharton's Jelly) (E: insert).
D2-40 is expressed in villous stroma throughout pregnancy
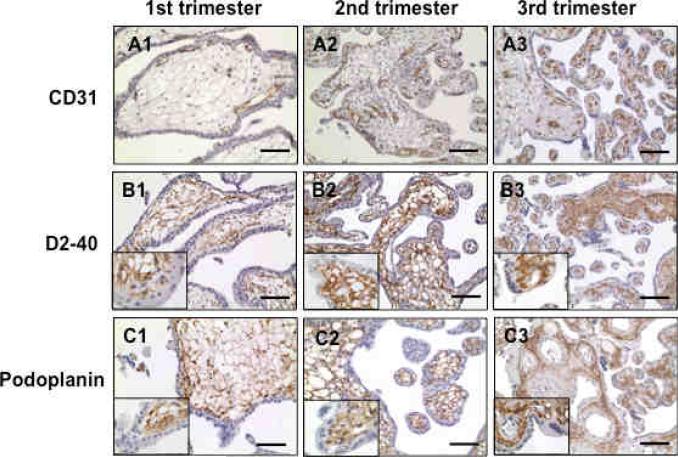
We next examined if D2-40 was differentially expressed in placentas from early versus later gestational stages. Tissue sections from 1st, 2nd, and 3rd trimester placentas were stained with D2-40 and CD31 antibodies. To verify D2-40 staining, antibody specifically against human podoplanin was also used since D2-40 specifically recognizes human podoplanin. Our results showed that D2-40 was expressed in villous stroma in all tissues that we examined, 4 from 1st trimester (8-10 weeks), 4 from 2nd trimester (16-18 weeks), and 4 from 3rd trimester (37-38 weeks). Podoplanin staining revealed the same pattern as D2-40, localized in villous stroma, whereas CD31 was only expressed in villous core fetal vessels (Figure 3). Inserts in the B and C series show a higher magnification of D2-40 and podoplanin staining (1,000x), respectively. In addition, we also noticed that in the 1st trimester placenta, fetal vessels with positive CD31 staining are close to the cytotrophoblasts in villous core tissues (Figure 3A1), which suggest that factors produced by cytotrophoblasts might play a role in placental vascular development.
Figure 3.
Immunostaining of CD31, D2-40, and podoplanin in 1st, 2nd, and 3rd trimester placentas. Note positive CD31 staining in villous core vessel endothelium. The network pattern of D2-40 staining is seen in villous stromal throughout pregnancy. Podoplanin expression exhibits the same pattern as D2-40 in villous stromal throughout pregnancy. Bar = 100μm. Insert in the B and C series shows a higher magnification of D2-40 and podoplanin staining (1,000x), respectively.
Reduced D2-40 expression in placentas from preeclampsia
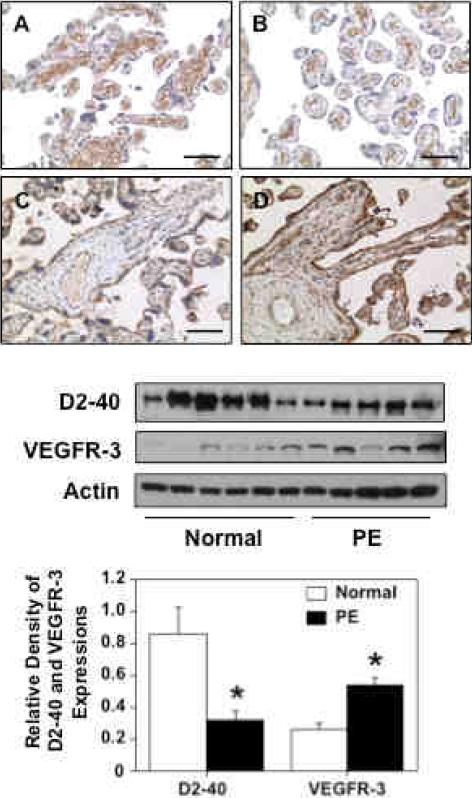
We next examined if D2-40/podoplanin expression was altered in preeclamptic placentas compared to placentas from normotensive pregnant controls. D2-40 and VEGFR-3 expressions were determined by immunohistochemical staining of tissue sections and by Western blot of whole tissue homogenate samples. A total of 11 samples were examined (6 from normotensive and 5 from preeclamptic pregnancies). Patient clinical information is shown in Table 1. Figure 4 upper panel shows representative D2-40 and VEGFR-3 immunostaining in placental tissue sections from normotensive and preeclamptic pregnancies. D2-40 immunoreactivity is localized in the stromal area of villous tissue sections and D2-40 expression is markedly reduced in the preeclamptic tissue sections (Figure 4B) compared to the normotensive tissue sections (Figure 4A). VEGFR-3 immunoreactivity is localized in both trophoblasts and villous core vessel endothelium. In contrast to D2-40 expression, VEGFR-3 expression is markedly increased in preeclamptic placental tissues (Figure 4D) compared to normotensive tissue sections (Figure 4C). Figure 4 lower panel shows D2-40 and VEGFR-3 protein expressions by Western blot. β-actin expression was also determined as the internal control for each sample. Relative band densities for D2-40 and VEGFR-3 expressions after normalized by β-actin expression are shown in Figure 4 lower panel bar graph. The results of immunostaining and Western blot are consistent in that D2-40 expression was significantly reduced but VEGFR-3 expression was significantly increased in preeclamptic placentas compared to those from normotensive controls.
Table 1.
Demographic data in normotensive and preeclamptic pregnancies
| Variables | Normal (n=6) | Preeclampsia (n=5) | p value |
|---|---|---|---|
| Maternal age (years) | 28 ± 6 | 23 ± 6 | 0.193 |
| Racial Status | |||
| White | 0 | 1 | --- |
| Black | 5 | 4 | --- |
| Other | 1 | ||
| Gestational Age (weeks) | 30 ± 8 | 33 ± 4 | 0.504 |
| BMI (at admission) | 31 ± 5 | 37 ± 8 | 0.175 |
| Blood Pressure (mmHg) | |||
| Systolic | 113 ± 14 | 175 ± 12 | <0.001 |
| Diastolic | 69 ± 11 | 101 ± 6 | <0.001 |
| Mode of Delivery | |||
| Vaginal | 4 | 0 | --- |
| Caesarean Section | 2 | 5 | --- |
Data presented as mean ± SD.
Figure 4.
Expressions of D2-40 and VEGFR-3 in placentas from normotensive and preeclamptic pregnancies. Upper panel: A and B are representative immunostaining of D2-40 and C and D are representative immunostaining of VEGFR-3 in normal and PE placentas. Bar=100μM. Lower panel: Western blot of D2-40 and VEGFR-3 expressions in normal (n=6) and PE (n=5) placental tissues. The bar graph shows the band density of Western blots. The immunostaining results and Western blot data are consistent that D2-40 expression was significantly reduced and VEGFR-3 expression was significantly increased in placentas from PE compared to normotensive pregnant controls, * p<0.05, respectively.
Discussion
D2-40 is a monoclonal antibody directed against human podoplanin, a transmembrane mucoprotein that is expressed in lymphatic endothelial cells. D2-40 reacts with an O-linked sialoglycoprotein (MW 40K) found on lymphatic endothelium, but does not react with blood vessel endothelium8. Thus, D2-40 has been used as lymphatic endothelial marker to study lymph-angiogenesis in physiological and pathological tissue samples and has been proved to be helpful in determining lymphatic invasion in tumor tissues9-12. Using D2-40 as a lymphatic marker, we examined D2-40/podoplanin expressions in the human placenta. Complementary immunostaining of blood vessels was obtained with established endothelial marker CD31. VEGFR-3 expression, a lymphatic-selective endothelial marker, was also examined for comparison. Interestingly, we found compartmental differences for D2-40 (podoplanin), CD31, and VEGFR-3 distribution in the placental villous tissue. D2-40 localized in the stroma exhibited a network plexiform distribution, CD31 was found in endothelium of villous core fetal vessels, and VEGFR-3 was localized to both trophoblasts and fetal vascular endothelium. The network pattern for D2-40 (podoplanin) expression in the placental stroma was additionally confirmed by examining D2-40 (podoplanin) expression in the 1st, 2nd, and 3rd trimester placental tissue sections (Figure 3). We found evidence for abundant expression of podoplanin in the normal placental tissue.
Podoplanin/D2-40 is a mucin-type transmembrane glycoprotein which was originally found on the surface of rat glomerular epithelial cells (podocytes), loss of podoplanin has been linked to the flattening of foot processes that occurs in glomerular diseases13. Podoplanin shows features of a membrane mucoprotein with conserved O-glycosylation sites. Although the precise biological function of podoplanin is not clear, podoplanin is known to be one of the most highly expressed lymphatic-specific transcripts in human lymphatic endothelial cells14. Therefore, D2-40/podoplanin expression has been used to define lymphatic vascular density in tumor tissues associated with lymphatic metastasis and poor prognosis. One study has also shown that heavily O-glycosylated mucoproteins probably function as counter-receptors for adhesion molecules like selectins which mediate cell attachment15, indicating mucoproteins may have important cell adhesion functions. While the placenta has no proven lymphatic vessels, the placenta has a high density blood vascular supply constantly undergoing dynamic angiogenesis throughout the first, second and early third trimesters. A well-functioning, fully developed placenta ensures a healthy fetus and a successful pregnancy. Although the biological function of podoplanin in the placenta is not known, based on the assigned role of this glycoprotein in interstitial fluid balance, placental podoplanin may support fetal vessel angiogenesis during placenta development within the villous stroma.
Placental villous tissue contains abundant mesenchymal and matrix channels, especially in the immature intermediate villi. Placental villous stroma is a unique channel-like structure16. It consists of a network of cells and fibers with fetal vessels. Within the stroma, it forms a fluid compartment with Hofbauer cells suspended in the interspaces16. Mesenchymal and matrix channels provide a path for Hofbauer cells to patrol the villous core. The distribution of D2-40/podoplanin in the stroma suggests that a lymphatic-like conductive network might exist in the human placenta. In addition to lymphatic endothelial cells and kidney podocytes, podoplanin is also expressed in germ cells, mesothelial cell, stromal reticular cells and many types of tumor tissues17. At present, we did not know as to what cells (Hofbauer cells/macrophages or fibroblasts) produce or secrete podoplanin in the placenta, even though we did notice D2-40 positive cells within villous stroma. Further studies to identify the cell type(s) that express and produce D2-40/podoplanin would be worth pursuing. Nonetheless, the network plexus pattern of D2-40/podoplanin in the placenta villous core stroma is highly unique, since none of the matrix proteins (including laminin α1, α2, α3, α5, β1, β2, and γ1 chains and extradomain A, extradomain B, and oncofetal fibronectin) showed a similar distribution pattern as to podoplanin18. Although D2-40/podoplanin is a marker for lymphatic endothelium, our data suggest that cells other than lymphatic endothelial cells express D2-40 within the placenta.
Interestingly, we found reduced D2-40/podoplanin expression in placentas from women with preeclampsia compared to those from normotensive pregnant controls. Reduced D2-40 expression is demonstrated by both immunostaining of villous tissue sections and by total protein expression of snap frozen placental tissue homogenates. It is well known that abnormal angiogenesis occurs in the placenta during preeclampsia. This has been demonstrated by reduced expression and production of placental growth factor (PlGF) and vascular endothelial growth factor (VEGF-A) and increased expressions and production of anti-angiogenic factors of sFlt-1 and sEng19, 20. Defective trophoblast invasion and angiogenesis are believed to contribute significantly to the placental pathophysiology in preeclampsia.
Increased VEGFR-3 expression is another finding of the present study. VEGFR-3 is also considered a marker for lymphatic endothelium. In contrast to D2-40, we found that VEGFR-3 expression is localized in trophoblast cells and in villous core vessel endothelium. The pattern of VEGFR-3 expression is similar to LYVE-1 expression in the placental villous tissue as we previously reported4. In addition, we also found increased VEGFR-3 expression in preeclamptic placentas compared to those from normotensive controls. The reason for increased VEGFR-3 expression in preeclamptic placentas is not clear. However, this could be linked to the hypoxia condition that is believed to be associated with placental pathophysiology in preeclampsia. Although direct evidence of VEGFR-3 up-regulation by hypoxia in the placenta is lacking, an in vitro study did show that hypoxia could promote VEGFR-3 expression in MCF7 and A549 adenocarcinomal cells21. In addition, hypoxia-driven vascular development requiring the activity of VEGFR-3 has also been demonstrated during mouse embryonic stem cell differentiation22. Increased VEGFR-3 expression could be a compensative mechanism to overcome increased oxidative stress and promote placental angiogenesis in the preeclamptic placenta.
At present, the outcome of reduced D2-40 in preeclamptic placenta is not clear. However, reduced D2-40 expression in quantity and quality may contribute to placental dysfunction in preeclampsia. Reduced D2-40 expression may lead to failure of interstitial fluid conduction, and subsequently increase in interstitial fluid accumulation, pressure, and edema. Poor placental tissue perfusion in preeclampsia might provoke higher perfusion pressures, which are pathognomonic for preeclampsia. Whether reduced D2-40/podoplanin sialoglycoprotein expression and/or dysregulation of the protein function contribute to improper trophoblast invasion, abnormal placental angiogenesis, and maladaptive immunity in preeclamptic placenta warrant further investigation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Red-Horse K, Rivera J, Schanz A, Zhou Y, Winn V, Kapidzic M, Maltepe E, Okazaki K, Kochman R, Vo KC, Giudice L, Erlebacher A, McCune JM, Stoddart CA, Fisher SJ. Cytotrophoblast induction of arterial apoptosis and lymphangiogenesis in an in vivo model of human placentation. J Clin Invest. 2006;116:2643–2652. doi: 10.1172/JCI27306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Red-Horse K. Lymphatic vessel dynamics in the uterine wall. Placenta. 2008;29:S55–59. doi: 10.1016/j.placenta.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weller RO, Djuanda E, Yow HY, Carare RO. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009;117:1–14. doi: 10.1007/s00401-008-0457-0. [DOI] [PubMed] [Google Scholar]
- 4.Gu B, Alexander JS, Gu Y, Zhang Y, Lewis DF, Wang Y. Expression of lymphatic vascular endothelial hyaluronan receptor-1 (LYVE-1) in the human placenta. Lymphat Res Biol. 2006;4:11–17. doi: 10.1089/lrb.2006.4.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey D, Baumal R, Law J, Sheldon K, Kannampuzha P, Stratis M, Kahn H, Marks A. Production of a monoclonal antibody specific for seminomas and dysgerminomas. Proc Natl Acad Sci U S A. 1986;83:5291–5295. doi: 10.1073/pnas.83.14.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marks A, Sutherland DR, Bailey D, Iglesias J, Law J, Lei M, Yeger H, Banerjee D, Baumal R. Characterization and distribution of an oncofetal antigen (M2A antigen) expressed on testicular germ cell tumours. Br J Cancer. 1999;80:569–578. doi: 10.1038/sj.bjc.6690393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao S, Gu Y, Lewis DF, Wang Y. Predominant basal directional release of thromboxane, but not prostacyclin, by placental trophoblasts from normal and preeclamptic pregnancies. Placenta. 2008;29:81–88. doi: 10.1016/j.placenta.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukunaga M. Expression of D2-40 in lymphatic endothelium of normal tissues and in vascular tumours. Histopathology. 2005;46:396–402. doi: 10.1111/j.1365-2559.2005.02098.x. [DOI] [PubMed] [Google Scholar]
- 9.Kahn HJ, Marks A. A new monoclonal antibody, D2-40, for detection of lymphatic invasion in primary tumors. Lab Invest. 2002;82:1255–1257. doi: 10.1097/01.lab.0000028824.03032.ab. [DOI] [PubMed] [Google Scholar]
- 10.Kimura N, Dota K, Araya Y, Ishidate T, Ishizaka M. Scoring system for differential diagnosis of malignant mesothelioma and reactive mesothelial cells on cytology specimens. Diagn Cytopathol. 2009;37:885–890. doi: 10.1002/dc.21128. [DOI] [PubMed] [Google Scholar]
- 11.Raica M, Cimpean AM, Ribatti D. The role of podoplanin in tumor progression and metastasis. Anticancer Res. 2008;28:2997–3006. [PubMed] [Google Scholar]
- 12.Dumoff KL, Chu CS, Harris EE, Holtz D, Xu X, Zhang PJ, Acs G. Low podoplanin expression in pretreatment biopsy material predicts poor prognosis in advanced-stage squamous cell carcinoma of the uterine cervix treated by primary radiation. Mod Pathol. 2006;19:708–716. doi: 10.1038/modpathol.3800580. [DOI] [PubMed] [Google Scholar]
- 13.Breiteneder-Geleff S, Matsui K, Soleiman A, Meraner P, Poczewski H, Kalt R, Schaffner G, Kerjaschki D. Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am J Pathol. 1997;151:1141–1152. [PMC free article] [PubMed] [Google Scholar]
- 14.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasseti C, Tangemann K, Singer MS, Kershaw DB, Rosen SD. Identification of podocalyxin-like protein as high endothelial venule ligand for l-selectin: parallels to CD34. J Exp Med. 2000;187:1965–1975. doi: 10.1084/jem.187.12.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufmann P, Stark J, Stegner HE. The villous stroma of the human placenta. Cell Tissue Research. 1977;177:105–121. doi: 10.1007/BF00221122. [DOI] [PubMed] [Google Scholar]
- 17.Ordóñez NG. Podoplanin: a novel diagnostic immunohistochemical marker. Adv Anat Pathol. 2006;13:83–88. doi: 10.1097/01.pap.0000213007.48479.94. [DOI] [PubMed] [Google Scholar]
- 18.Korhonen M, Virtanen I. Immunohistochemical localization of laminin and fibronectin isoforms in human placental villi. J Histochem Cytochem. 2001;49:313–322. doi: 10.1177/002215540104900305. [DOI] [PubMed] [Google Scholar]
- 19.Gu Y, Lewis DF, Wang Y. Placental productions and expressions of soluble endoglin, soluble fms-like tyrosine kinase receptor-1, and placental growth factor in normal and preeclamptic pregnancies. J Clin Endocrinol Metab. 2008;93:260–266. doi: 10.1210/jc.2007-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simiantonaki N, Jayasinghe C, Michel-Schmidt R, Peters K, Hermanns MI, Kirkpatrick CJ. Hypoxia-induced epithelial VEGF-C/VEGFR-3 upregulation in carcinoma cell lines. Int J Oncol. 2008;32:585–592. [PubMed] [Google Scholar]
- 22.Nilsson I, Rolny C, Wu Y, Pytowski B, Hicklin D, Alitalo K, Claesson-Welsh L, Wennström S. Vascular endothelial growth factor receptor-3 in hypoxia-induced vascular development. FASEB J. 2004;18:1507–1515. doi: 10.1096/fj.03-1276com. [DOI] [PubMed] [Google Scholar]