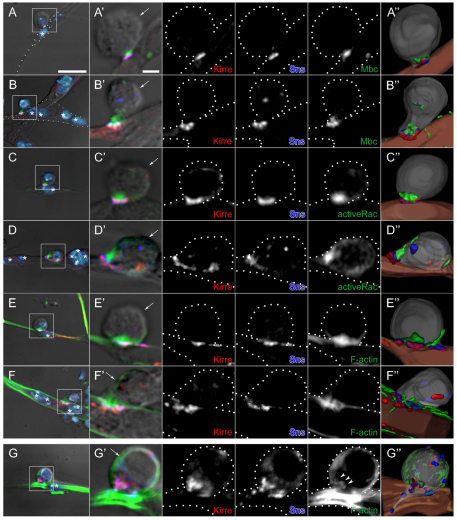
Fig. 4.
Localization of Mbc, active Rac1 and F-actin in fusion-competent myoblasts at points of contact with founder cells and myotubes in primary myoblasts. (A-G′) Primary myoblasts 14 hours after plating were immunostained for Kirre (red), Sns (blue) and either Mbc (A-B′), active Rac1 (C-D′) or F-actin (E-G′), shown in green. (A-F′) Single confocal sections of wild type; strong enrichment of all three proteins (Mbc, active Rac1, F-actin) in the fusion-competent myoblasts (FCMs). (G) DIC and projection images of four serial confocal sections from mbcD11.2 cells; absence of compact foci. A-G are lower magnification views that include DAPI nuclear staining (light blue). Boxed areas indicate the region shown at higher magnification in A′-G′. (A,C,E,G) Representative FCMs in contact with mononucleate founder cells. (B,D,F) FCMs in contact with syncitia of four to six nuclei. The FCM of each cell pair was identified by its rounded morphology visible by DIC optics and by post-fixation fluorescence of the native protein from an sns-mCherryNLS transgene (not shown). Founder cells/myotubes were identified by their elongated morphology (A,C,E) or the presence of multiple nuclei by DAPI staining (B,D,F). Asterisks indicate myotube nuclei and arrows indicate the FCM. Broken lines in single channel views are provided as a reference for cell boundaries. (A″-G″) Images from 3D reconstructions of z-series data. See Movies 1-4 in the supplementary material. Scale bars: 10 μm in A-G; 2 μm in A′-G′.