Figure 3.
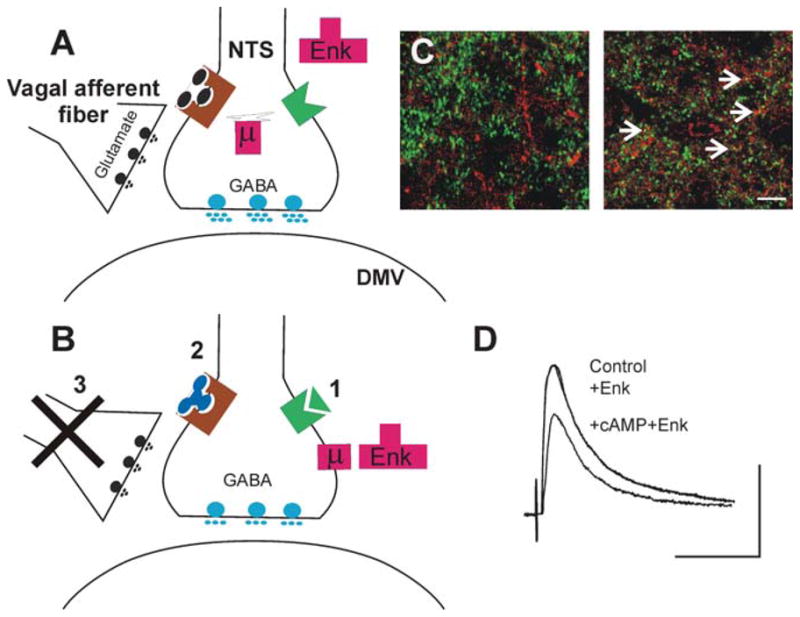
Increasing the levels of cAMP in the brainstem induces receptor trafficking in NTS nerve terminals. (A) In control conditions, i.e., when the levels of cAMP are low either because the tonic release of glutamate from vagal afferent fibers activates group II metabotropic glutamate receptors (left) or because Gαpled receptors are not activated (right), the terminals of GABAergic neurons in the NTS store μ-opioid receptors (μ) in internal compartments associated with the Golgi complex. In this situation, μ-opioid agonists (e.g., Enk) cannot modulate the release of GABA onto DMV neurons. (B) Following increases in cAMP levels within the GABAergic nerve terminal, for example, by (1) activation of a receptor coupled to Gα., TRH or CCK, (2) antagonism of group II metabotropic glutamate receptors, or (3) removal of tonic vagal afferent input, μ-opioid receptors are released from the Golgi apparatus and translocated to the nerve terminal membrane, where opioid agonists can inhibit GABA synaptic transmission between the NTS and the DMV. (C) High-power photomicrographs of the cloned μ-opioid receptor (MOR1)-immunoreactivity (-IR; TRITC filters, red) and glutamic acid decarboxylase-IR (GAD-IR; FITC filters, green) used as a marker for GABAergic nerve terminals in the rat DVC. In control conditions (left panel), note the absence of MOR- and GAD-IR colocalized profiles. Following vagal afferent rhizotomy (right panel), many nerve terminals show MOR- and GAD-IR colocalized profiles (yellow; arrows). Images represent three-dimensional reconstructions from Z-stack image series. Scale bar: 10 μm. (D) Representative traces of eIPSCs evoked in a gastric-projecting DMV neuron voltage-clamped at −50 mV. Perfusion with Enk does not affect the amplitude of IPSCs evoked by electrical stimulation of the NTS. Following five minutes’ perfusion with substances that increase the cAMP levels, however, reapplication of Enk reduces the amplitude of the eIPSCs. Scale bar: 50 ms and 200 pA.
