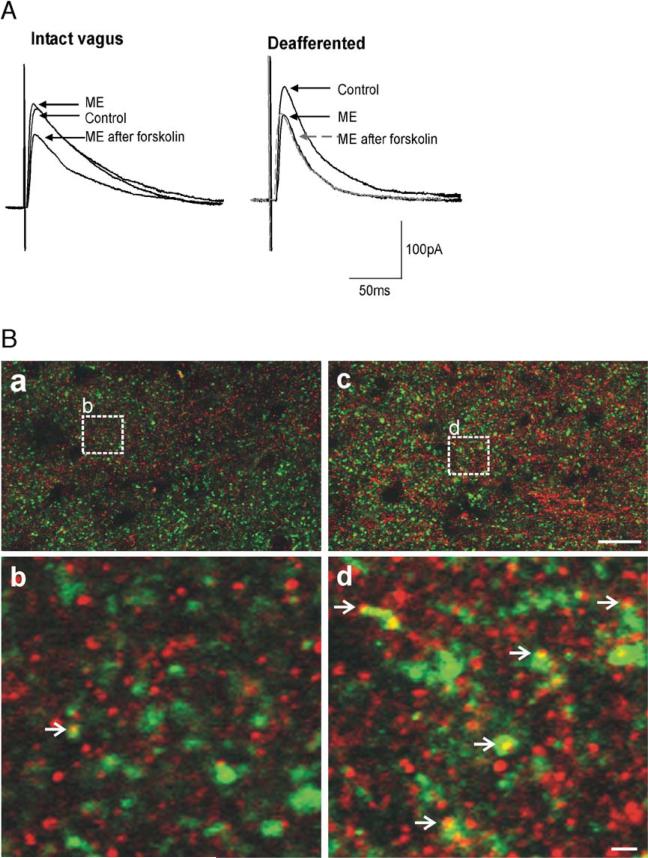
Fig. 2.
Vagal afferent input controls cAMP levels in GABAergic nerve terminals. A: In a gastric-projecting DMV neuron from a vagally intact rat, ME (10μM) induced an inhibition in eIPSC amplitude only following activation of the adenylate cyclase pathway (e.g. superfusion with forskolin (10μM) as shown; left). In contrast, in a rat in which the vagal afferent nerves were removed either chemically (perivagal capsaicin application) or surgically (afferent rootlet rhizotomy), ME (10μM) inhibited eIPSC amplitude without the need for prior activation of adenylate cyclase (right). Indeed, further activation of adenylate cyclase (e.g. forskolin, 10μM) resulted in no further inhibition by ME. B: Vagal deafferentation increases expression of μ-opioid receptor (MOR) on GABAergic profiles. MOR-IR: red; Glutamic acid decarboxylase-IR (GAD-IR; used to label GABAergic nerve terminals): green; profiles double-labeled for MOR and GAD: yellow. Panel a. Low magnification micrograph illustrating a portion of the DVC from the control (vagally intact) side of the brainstem. Panel b. High magnification micrograph illustrating the detail of a MOR-IR profile co-localized with GAD-IR (open arrowhead). The magnification is insert “b” shown in panel A. Panel c. Low magnification, micrograph illustrating a portion of the DVC from the contralateral (deafferented) side of the same sliceasin Panel A. Panel d. High magnification micrograph illustrating the detail of several MOR-IR profiles co-localized with GAD-IR (open arrowhead). The magnification is insert “d” shown in panel B. Scale bar=20μm in a and c; 2μm in b and d.