Abstract
Hyponatremia is a predictor of poor cardiovascular outcomes during acute myocardial infarction and in the setting of preexisting heart failure [1]. There are no definitive mechanisms as to how hyponatremia suppresses cardiac function. In this report we provide evidence for direct down-regulation of Ca2+ channel current in response to low serum Na+. In voltage-clamped rat ventricular myocytes or HEK 293 cells expressing the L-type Ca2+ channel, a 15 mM drop in extracellular Na+ suppressed the Ca2+ current by ~15%; with maximal suppression of ~30% when Na+ levels were reduced to 100 mM or less. The suppressive effects of low Na+ on ICa, in part, depended on the substituting monovalent species (Li+, Cs+, TEA+), but were independent of phosphorylation state of the channel and possible influx of Ca2+ on Na+/Ca2+ exchanger. Acidification sensitized the Ca2+ channel current to Na+ withdrawal. Collectively our data suggest that Na+ and H+ may interact with regulatory site(s) at the outer recesses of the Ca2+ channel pore thereby directly modulating the electro-diffusion of the permeating divalents (Ca2+, Ba2+).
Keywords: L-type Ca2+ channel, Na+-regulation, cardiac myocytes, ion channels, PKA phosphorylation, Ca2+ channel agonists, Bay K 8644
1. Introduction
Voltage-gated L-type Ca2+ channels are essential regulators of a number of cardiac functions as they provide the critical trans-membrane influx of Ca2+ that triggers the release of Ca2+ from the sarcoplasmic reticulum (SR) for activation of contraction. L-type Ca2+ channels have been extensively studied in terms of their permeability, selectivity, gating, molecular structure and regulation. Despite high selectivity of these channels to Ca2+, earlier studies reported significant permeability also to other divalents and monovalents, especially Na+ in absence of Ca2+ [2]. In addition, a number studies have shown suppression of ICa on withdrawal of Na+ in the presence of Ca2+ consistent, perhaps, with significant Na+ permeability of the Ca2+ channel [3–6]. This effect appeared to depend on the substituting monovalent cations such that while replacement of Na+ with Cs+, TEA+ and Li+ reduced ICa significantly, substitution by TrisCl had little or no effect on ICa [7]. Studies substituting equi-osmolar sucrose or mannitol for NaCl were inconclusive as they destabilize the myocytes [8–11]. The two mechanisms proposed for low Na+ suppressive effects on ICa were: 1) Inactivation of Ca2+ channels secondary to influx of Ca2+ on the Na+/Ca2+ exchanger (NCX), on withdrawal of Na+ [5]; and 2) Significant permeation of Na+ through the channel in the presence of Ca2+. The first mechanism appears to be unlikely as Na+ removal continues to suppress the channel even when Ba2+ is the charge carrier [7, 11]. The most compelling evidence against the second, direct Na+ permeation, possibility was the finding that 1 μM of extracellular Ca2+ blocked the permeation of high concentrations of monovalents, consistent with high selectivity (>1/10,000) of the channel to Ca2+ [3, 12–13].
More recent reports provide yet another possibility that positive or negative regulation of channel current by extracellular cations results from their binding and electrostatic interactions with specific pore-forming residues of the channel, as for instance described for the voltage-dependent blockade of Na+ channels by Ca2+ [14], or electrostatic interactions of extracellular protons with glutamate residues of Ca2+ channels that mediate the suppressive effects of proton on ICa [15–16].
The aim of our study was to examine the low Na+ suppressive effects on ICa in terms of direct interaction of Na+ with the Ca2+ channel pore. We hypothesized that interaction of Na+ within the channel pore, in the presence of Ca2+, may increase the open probability of the channel, thus enhancing Ca2+ permeation of the channel. We have examined the effect of Na+ withdrawal on whole cell ICa using both rat ventricular myocytes and HEK 293 cells expressing the recombinant L-type Ca2+ channel. In addition to reexamining the previous hypotheses regarding substituted monovalent species and possible influx of Ca2+ on NCX, we used several novel approaches that include: Quantifying the concentration- and voltage-dependent effects of Na+ withdrawal on ICa; examining the efficacy of ICa suppression as the channel mean open-time was increased by (s)-(−) Bay K 8644, phosphorylation, or substituting Ba2+ for Ca2+ as the permeant divalent; and determining possible overlapping functions of Na+ and H+ at extracellular regulatory sites associated with the channel pore.
Our data support the possibility that extracellular Na+ modulates the Ca+ channel by displacing H+ from a low affinity binding sites, in the electrostatically crowded environment of the channel pore.
2. Methods
2.1. Enzymatic isolation of Cardiac myocytes
Cardiac cells were isolated from adult male Wistar rats (200–300 g) through enzymatic digestion of cardiac tissue as described previously [17]. All animal experiments were conducted in accordance with Georgetown University Animal Care and Use Committee. Rats were anesthetized by i.p. injection of sodium pentobarbital (150 mg/kg) after which the heart was rapidly excised from the chest cavity and transferred to a Langendorff apparatus to undergo retrograde perfusion through the aorta at a rate of 7 ml/min. Initially, a Ca2+-free Tyrode’s solution (In mM: 137 NaCl, 5.4 K L-glutamate, 10 HEPES, 1 MgCl2, 10 Glucose, pH 7.35 at 37 °C) was perfused for 6 min followed by the same Ca2+ free solution containing collagenase (1.4 mg/ml; Roche) and protease (0.16 mg/ml; Sigma-Aldrich) for 13 min and a final 6 min perfusion with the Tyrode’s solution containing 0.2 mM CaCl2. The right and left ventricles of the digested heart were then cut into several sections and single cells were isolated by gently agitating the tissue. Cells were stored on cover slips at room temperature in 0.2 mM CaCl2 Tyrode’s solution. Following a cell adhesion period (~ 30 min), the cover slips were transferred to the stage of an inverted Nikon microscope for patch-clamp experiments.
2.2. Transient Transfections
Recombinant Ca2+ channel subunits α1C,77-YFP, β1, and α2δ were a generous contribution of Dr. Nikolai Soldatov (NIA, Baltimore MD). HEK 293 cells were grown in 6-well plates using Dulbecco’s Modified Eagle Medium with 10% fetal bovine serum at 90% confluence. Using Lipofectamine™ 2000 (Invitrogen) gene transfection assay, 1–6 μg of each subunit was mixed with a standard amount of lipofectamine reagent and incubated for about 20 minutes. After incubation, the mixture was transferred to each well while swirling the plates. Cells were then incubated for 24 hours allowing for gene expression to take place. The next day, cells were split onto cover slips at 10% confluence and allowed to incubate for another 48 hours before being used for patch-clamp studies. Gene expression was determined at a rate of 60–70% using a GFP fluorescence filter (Chroma Technology) for excitation/emission at 470/525 nm. The GFP-filter cube was inserted into the epi-fluorescence light pass of the Nikon Diaphot inverted microscope and illuminated by a RatioArc mercury lamp. Cells expressing green fluorescence were used for patch-clamp experiments.
2.3. Electrophysiology
Ca2+ current (ICa) measurements
Patch pipettes (2–5 MΩ) were filled with an internal solution containing (mM): 120 CsOH, 110 aspartic acid, 20 TEACl, 2 EGTA, 5 MgATP, 20 HEPES, titrated to pH 7.2. In some experiments 200 μM cAMP was added to the pipette solution to fully activate PKA phosphorylation. Cells were voltage-clamped at a holding potential of −50 mV to inactivate Na+ channels. Peak ICa was measured using 25–50 ms depolarization pulses to 0 or 10 mV. For current-voltage (I-V) relationships we used test potentials ranging from −40 to +80 mV with 10 mV increments. Steady-state availability was measured by varying a 500 ms preconditioning potential from −80 mV to +30 mV, followed by a test pulse to +10 mV. Activation plots were measured by normalizing I-V currents to peak value at ~0 mV for each cell before and after solution exchange. Activation and availability plots were individually fitted using a standard Boltzmann function:
where V0.5 and the slope factor, Vc, were obtained using Origin6.0 software. All records were bracketed before and after solutions exchange to obtain individual baseline values for each cell. Currents were recorded using a Dagan amplifier and pClamp 5.5 software (Axon Instruments). Data were analyzed and plotted using Origin6.0 and pClamp 9.0 software. Currents were paired with baseline values before each intervention and the means were compared using a paired Student’s t-test. ANOVA was used to compare group means. Results are shown as mean ± S.E.
2.4. Chemicals and Solutions
The standard extracellular Tyrode’s solution contained (mM): 145 NaCl, 1 MgCl2, 10 HEPES, 10 glucose, and 2 CaCl2. pH was titrated to 7.4 using NaOH. KCl was removed from external solutions to prevent IK activation. Reduced values of [Na+]o in the range from 130–5 mM were achieved by replacement of NaCl with equimolar concentrations of, CsCl, TEACl, or LiCl or equiosmolar concentrations of sucrose. In order to inhibit Na+/Ca+ exchanger from operating in the Ca2+-influx (reverse) mode under low [Na+] experiments, NaCl was removed from internal solutions. In addition, in some experiments we used 1–5 μM of tetrodotoxin (TTX; Alamone Laboratories) to block voltage-gated Na+ channels. (S)-(−)-Bay K 8644 was obtained from Sigma-Aldrich Inc. (St. Louis, MO). To characterize membrane responses we used a rapid solution exchange system well established in our laboratory and previously described [18]. In short, solutions were placed in a five barrel apparatus attached to electronically operated valves that connected through 5 separate manifolds into a single outflow tip located ~0.2 mm from the cell. Upon commanding the electronic unit, solution was discharged from the tip within 20 ms. Complete superfusion was achieved in ~50 ms. All experiments were carried out at room temperature.
3. Results
3.1. Exploring the effect of extracellular [Na+] on ICa
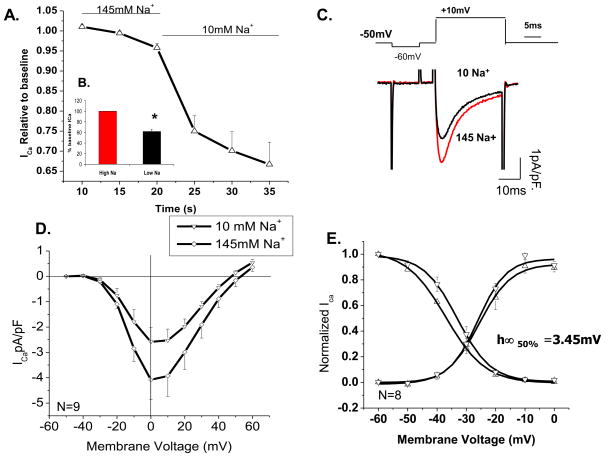
To examine the regulatory role of extracellular Na+ on ventricular L-type Ca2+ current, we reduced the extracellular [Na+] to10 mM from its control value of 145 mM, by replacing it with equimolar concentrations of Cs+. Figure 1 shows the effect of rapid (50 ms) reduction of external [Na+] to 10 mM on ICa activated by step depolarizations from −50 to 0 mV for 100 ms. In some experiments, in addition to a holding potential of −50 mV, 5–10 μM of TTX was required to fully suppress INa. Figure 1A shows that ICa decreased by 35 % within first three depolarizing pulses (representative currents, panel C) applied at 5 sec intervals, reducing average peak density of unphosphorylated ICa, at 0 mV, from −4.07 to −2.58 pA/pF (N=9, p=0.004; Fig. 1D). In all experiments, I-V protocols were applied following the single-step-pulse depolarization protocol, with at least one-minute interval between the two protocols. During this interval, ICa further decreased by about 5–10% (Fig. 1B), possibly related to slow exchange of the t-tubular space or ICa run down. To exclude the complicating factor of Ca2+ channel run down from data analysis, the suppressive effects of low Na+ were generally quantified only in the first 10 to 15 sec of low Na+ exposure. I-V relations in low Na+ were often shifted by ~ −10 mV in both the apparent reversal potential and the peak inward current. The activation and availability parameters of ICa, measured with test and conditioning pulse protocols, respectively, showed no significant change (p=0.79) in activation parameter, but there was a small statistically significant shift of −3.45 mV (p=0.039) in the half-maximal voltage (V0.5) of availability curve, with no change in the slope factor (p=0.74; Fig. 1E).
Figure 1. Reducing extracellular [Na+] leads to suppression of baseline unphosphorylated ICa.
A) Time course of peak ICa suppression under rapid fluid exchange to low (10mM) extracellular [Na+]. Currents were recorded under a single step depolarization to +10 mV from holding potential of −50 mV. B) Quantitative representation of peak ICa suppression in low [Na+]o solutions. C) Representative peak traces from high and low extracellular [Na+] in the same cell. D) I-V-relation of ICa in 145 (◇) and 10 mM (▽) extracellular [Na+] (N=9, p=0.004). E) Corresponding activation and availability plots at 145 mM Na+ (▽) and 10 mM Na+ (△) (N=8). For measurements of availability, the membrane was held for 500 ms at a conditioning potential ranging from −80 to +30 mV and followed by a depolarizing test pulse to +10mV.
3.2. The Low Na+ effect in PKA phosphorylated chnnels
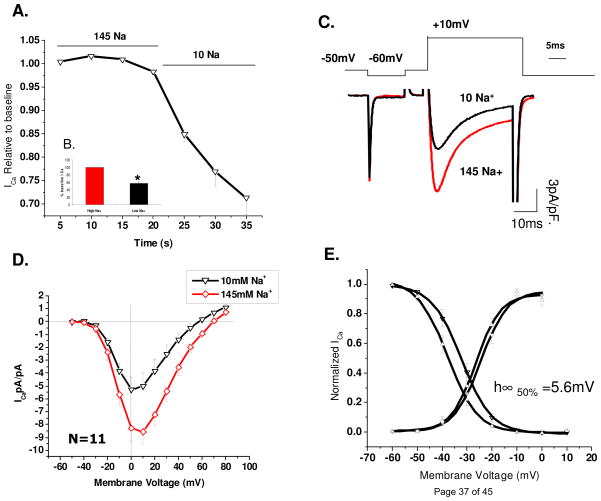
L-type Ca2+ channels are regulated by PKA phosphorylation leading to their increased mean open time, open probability and influx of permeating cations [5, 19–20]. To examine whether PKA-induced phosphorylation altered the low Na+ effect, 200 μM cAMP was included in the patch pipette solutions. Peak ICa in cAMP dialyzed cells ranged between 8–25 pA/pF as compared to 2–6 pA/pF in contol solutions. Figure 2 shows that ICa decreased by 33 % within the first 15 s of solution exchange, as compared to 43±4%, measured during more protracted recordings of I-V relations (Fig. 2AB). Similar suppressive effects were seen at all voltages tested. The activation curves were once again not significantly changed, but the availability parameters showed a statistically significant −5.6 mV (p=0.005) shift in V0.5 with no or minor change in the slope factor (p=0.98; panel 2E). These results suggest that the suppressive effects of low Na+ were not significantly altered by the phosphorylation state of the channel.
Figure 2. Reducing extracellular [Na+] leads to suppression of phosphorylated ICa.
A) Time course of peak ICa suppression under rapid fluid exchange to low (10 mM) extracellular [Na+]. B) Quantitative representation of peak ICa suppression in low [Na+]o solutions. C) Representative peak traces from high and low extracellular [Na+] in the same cell. D) I-V-relation of ICa in 145mM (◇) and 10mM (▽) extracellular [Na+] (N=11, p=0.007). E) Corresponding activation/availability plots measured with 145 mM (▽) or 10 mM [Na+]o (△; N=9).
3.4. Concentration-dependence of the low Na+ effect
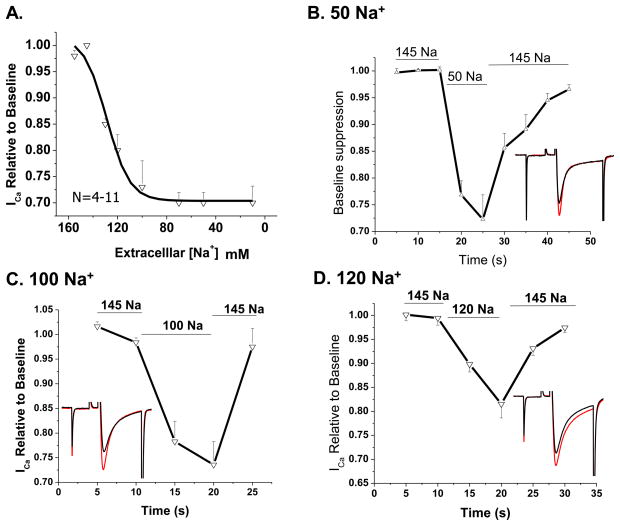
We used concentrations of Na+ ranging from 10–145 mM to determine the concentration-dependence of the low Na+ effect. Because the low Na+ effect was not significantly altered by channel phosphorylation, in this set of experiments 200 μM cAMP was included the patch pipette solutions to minimize the Ca2+ current run down. Figure 3 shows that ICa decreased as a function of reducing the [Na+]o from 145 to 70 mM, but the suppressive effect below 70 mM leveled off at 33% of the current in control solutions. ICa suppression was 25%, 20% and 15% with 100, 120 and 130 mM Na+ respectively, with an IC50 at 128 mM, when measured 10–15 s following reduction of Na+ (Fig. 3A). Figure 3B–D show cumulative time course of suppression of ICa seen in experiments with 10 s exposure to solution with 50, 100 and 120 mM [Na+]o (N=4–5). Increasing Na+ back to control levels recovered ICa to 95% of its baseline values within 5–15 seconds.
Figure 3. ICa suppression in low extracellular [Na+] is concentration dependent at [Na+]o above 100 mM.
A) Concentration-dependence of ICa suppression in various extracellular Na+ concentrations ranging from 145 to 10 mM (N=4–11, p<0.01 at all points). B–D) Time course of ICa suppression in extracellular solutions containing 50, 100, 120 mM NaCl respectively. Extracellular Na+ was replaced by equivalent amount of Cs+.
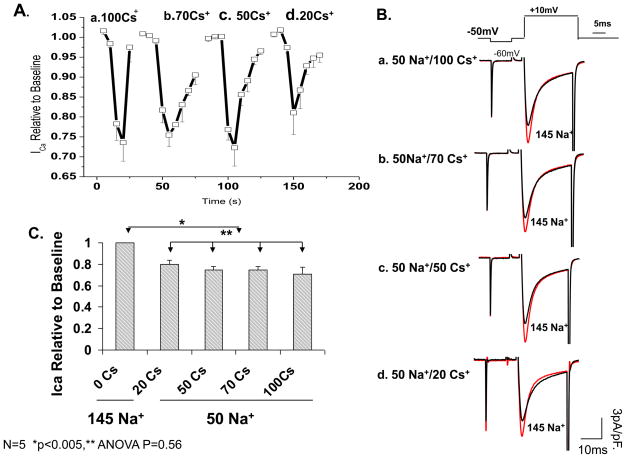
In the experiments of Figure 3, since Cs+ was used to replace Na+, we tested if differential surface charge effects between the two cations were responsible for the degree of the current suppression by varying the extracellular Cs+ concentrations from 20–100 mM while keeping the concentration of Na+ constant at 50 mM, using sucrose to compensate for the osmotic imbalance. Figure 4 shows that changing Cs+ concentration in the presence of 50 mM [Na]o neither altered the suppressive effect of low Na+ on ICa (ANOVA N=4, p=0.56; panel 4C), nor did it change its voltage-dependence, or gating parameters (data not shown). Our data, therefore, do not support significant role for differential surface charge screening effects of these two monovalents.
Figure 4. Extracellular Cs+ concentration does not affect the degree of ICa suppression by removal of extracellular Na+.
A) Cumulative timeline of ICa suppression in 50 mM extracellular Na+ using varying concentrations of CsCl 100 (a), 70 (b), 50 (c) and 20 mM (d) as the substituting cation and with sucrose added to maintain constant osmolarity. B) Representative currents from a single myocyte exposed to 50 mM external Na+ along with varying external CsCl (20–100mM, a–d). C) Quantitative representation of A in 4 cells. P<0.005 compared to control, P=0.56 between the various concentrations using a one way ANOVA.
3.5. Modulation of low Na+ effects on ICa by different monovalent substitutions
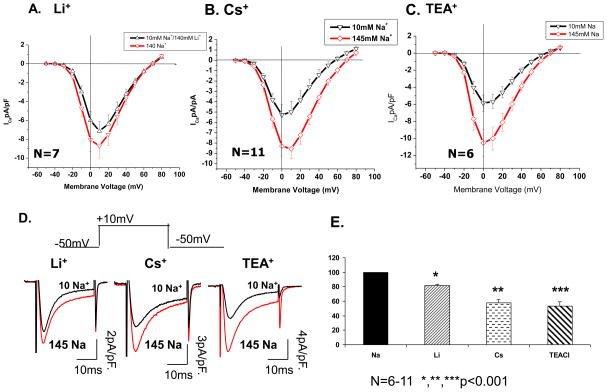
Figure 5A–C compares I-V relation of ICa obtained by replacing all but 10 mM NaCl with equivalent amounts of LiCl (A), CsCl (B) and TEACl (C). Consistently, LiCl decreased ICa by only 18% (from 8.7±1.3 to 7.1±1.1 pA/pF; N=7, p=0.002) as compared to 38% suppression by Cs+. TEA substitution produced the largest (~45%) suppression of ICa from 10.5±1.5 to 5.9±1.0 pA/pF (N=6, p=0.01; see also figure 5E). The smaller effect of substituting Li+ may be related to its comparable size to Na+, while TEA+, with its larger size and hydration shell, is least likely to replace Na+ within the outer vestibule of channel pore, and may thus produce the largest suppressive effect on ICa. Interestingly, only replacement of Na+ with Cs+, but not Li+ or TEA produced a small negative shift in the apparent reversal potential of ICa (compare panels A, B, & C of Fig. 5).
Figure 5. ICa suppression depends on the ionic properties of the extracellular monovalent cation.
A–C) I-V relation of ICa in external solutions containing 145mM (◇) and 10 mM (▽) NaCl using 140 mM of LiCl, CsCl or TEACl to replace the remaining NaCl. D) Representative traces of ICa suppression in 10 mM Na+ replacing the extracellular cation with Li+, Cs+ and TEA+. E) Quantitative representation of relative peak current suppression from baseline when extracellular monovalents were varied between Na+, Li+, Cs+ and TEA+ in 10 mM Na+ (N=6–15, p<0.01 for all three conditions).
3.6. The low Na+ effect on the recombinant L-type Ca2+ channel
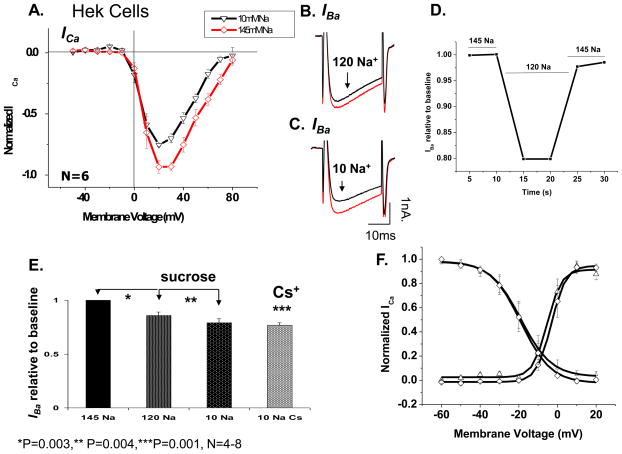
In order to validate the low Na+ suppressive effect as a property of the L-type channel protein, we examined this effect in HEK 293 cell expressing all the subunits of the L-type channel gene (α1C,77-YFP, β1, and α2δ). Figure 6A, shows I-V relation of ICa before and after exposure of the cell to 10 mM [Na+]o using Cs+ as the substituting cation for Na+. ICa was suppressed by 20% from its control values within 10 s of the exchange of extracellular solutions (N=6, p=0.001). When Ba2+ was the charge carrier through the channel withdrawal of Na+ similarly suppressed IBa.. Figure 6B–C shows representative traces of IBa suppression at the two concentrations of Na+, while Figure 6D depicts the time course of IBa suppression on reducing Na+ to 120 mM. Quantitative representation of data at the two concentrations using either sucrose or Cs+ (N=4 and 8 respectively) is shown in Figure 6E. Maximal suppression of ICa was 23% in 10 mM Na+ and 14% in 120 mM [Na]o. These values are slightly smaller than those observed in native cardiac myocytes, but do show similar suppressive effects of low Na+ on ICa. It is possible that the differences between native myocyte and recombinant Ca2+ channels are related to post translational modification such as glycosylation of the α2δ subunit [21–22].
Figure 6. Low extracellular Na+ concentration leads to ICa suppression in HEK 293 cells expressing recombinant Ca2+ channel.
A) Current-voltage relationship of ICa in 145mM (◇) and 10mM (▽) [Na]o in HEK 293 cells expressing α1c,77, β1, α2δ subunits of the L-type Ca2+ channel (N=6). B–C) Representative traces from the same cell after reduction of [Na]o to 120 mM (B) and 10 mM [Na]o (C) with equi-osmolar substitution of remaining NaCl with sucrose. D) Representative time course of suppression in 120 mM external Na+. E) quantitative representation of IBa suppression in two concentrations of 120 mM and 10 mM [Na+]o comparing substitution with both Cs+ and sucrose (N=5–6, p<0.004 for all three conditions). F) Activation and availability curves of the L-type Ca2+ channel in presence of 145 (◇) and 10 mM [Na]o (△) using Cs+ as the substituting cation.
3.7. Evaluating the Role of NCX and Localized CICR in the Low Na+ Effect
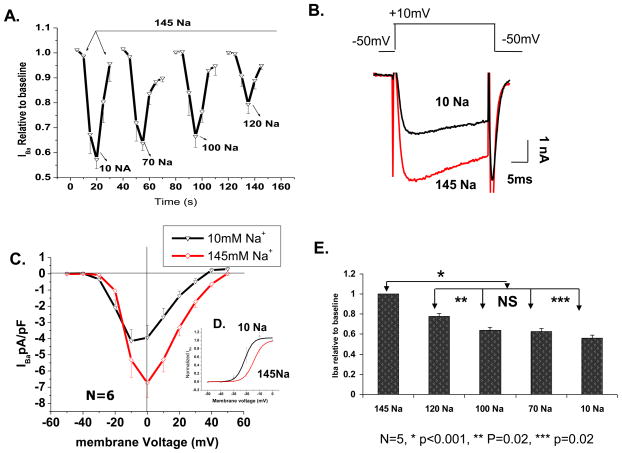
Entry of Ca2+ through the L-type Ca2+ channel not only inactivates the channel (CDI), but also induces SR Ca2+ release which further contributes to CDI of the channel [23–24]. Using weakly Ca2+-buffered internal solutions (2 mM EGTA), therefore we probed whether reducing the extracellular Na+, which enhances Ca2+ influx on NCX, might inactive ICa directly, or indirectly by activating Ca2+ release (CICR) from SR. This possibility was tested by repeating the above experiments using Ba2+ (known not to activate CICR, [25]) as the charge carrier through the Ca2+ channels. Figure 7A depicts the cumulative time course of this response at extracellular Na+ concentrations of 10, 70, 100 and 120 mM. Similar to the observed suppressive effects of low Na+ on ICa, Ba2+-transporting channels were also suppressed in a concentration dependent manner. Somewhat larger suppressions were noted in Ba2+ transporting channels: 44% in 10 mM Na+, 36% in 70 mM Na+ and 22% in 120mM Na+ (panel 7E). Interestingly, with Ba+ as charge carrier, a relatively larger shift in the apparent reversal potential (~−12 mV; panel 7C) and smaller shift in the activation curve (~−7 mV; panel 7D), were noted only when Cs+ substituted for Na+. The similarity of low Na+ suppressive effects on IBa and ICa rules out significant role for involvement of NCX and CICR in the low Na+ response.
Figure 7. Reducing extracellular Na+ leads to L-type IBa suppression in a similar fashion as ICa.
A) Cumulative time course of suppression in four external NaCl concentrations (mM) of 10 (a), 70 (b), 100 (c) and 120 (d) (N=5 at each concentration). The duration of each response is depicted on the time-axis. The arrow points to control and washout and the valleys represent peak amplitude suppression at the given Na+ concentration. B) representative Ba2+ current trace from a single myocyte exposed to 145 and 10 mM NaCl. C) I-V relation of IBa in 145mM (◇) and 10 mM (▽) NaCl extracellular solutions (N=6). D) Activation curve of IBa in high (◇) and low (△) extracellular Na+. E) Quantitative representation of (A).
3.8. Do longer mean open times enhance the low Na+ suppressive effects?
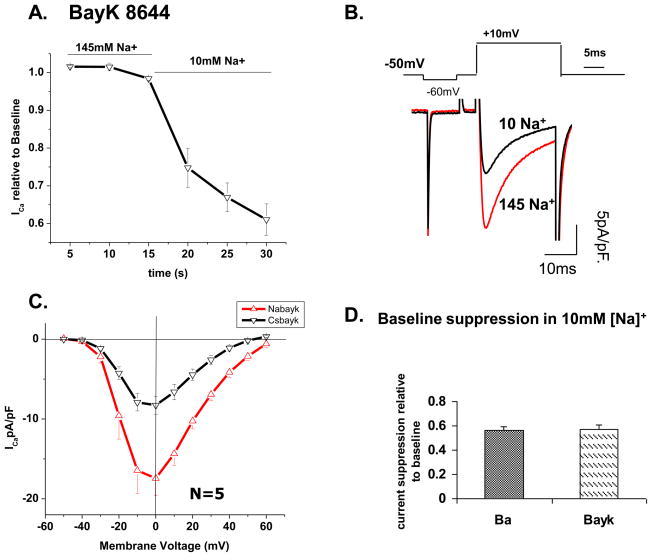
In support of this idea the low Na+ suppressive effects were larger (Fig. 7) when channel mean open times were prolonged by inhibiting CDI and CICR in Ba2+ transporting channels. We further probed this idea by using the Ca2+ channel agonist (S)-(−)-Bay K 8644, known to prolong the mean open time of the channel and to desensitize the channel to Ca2+ dependent inactivation [22, 26–28]. Application of 1 μM Bay K 8644 increased peak ICa from an average of 10 pA/pF to 17.4 pA/pF and slowed the inactivation kinetics by 53% at 0 mV (τ=5.1±1.9 vs. 10.8±3.1). Figure 8A shows a decrease in ICa of approximately 30% within the first 5 s of removal of Na+ and a maximal suppression 43% in ~15 s. Interestingly, the low Na+ suppressive effect in Bay K 8644 containing solutions (43%) was similar to that observed when Ba2+ was the charge carrier through the channel (44%). Peak ICa decreased from −17.4±2.2 to −8.3±1.4 pA/pF (N=5; panel 8C). These results suggest that prolonged mean open time of the channel or removing Ca2+ dependent inactivation sensitizes the channel to the effects of low [Na+]o.
Figure 8. ICa suppression in low Na+ is more pronounced when channels remain open with (S)-(−)-Bay K 8644.
A) Time course of ICa suppression in 10 mM extracellular Na+. Solutions were exchanged at 15 sec time point. B) Representative current trace from a cardiomyocyte incubated in 1 μM (S)-(−) Bay K 8644 before and after solution exchange to 10 mM [Na+]o. C) I-V relation of current suppression in high and low Na+ extracellular solutions containing 1 μM Bay K 8644. D) Quantitative comparison of ICa (in Bay K 8644) with IBa suppression in 10 mM extracellular Na+.
3.9. pH-dependence of the low Na+ effect
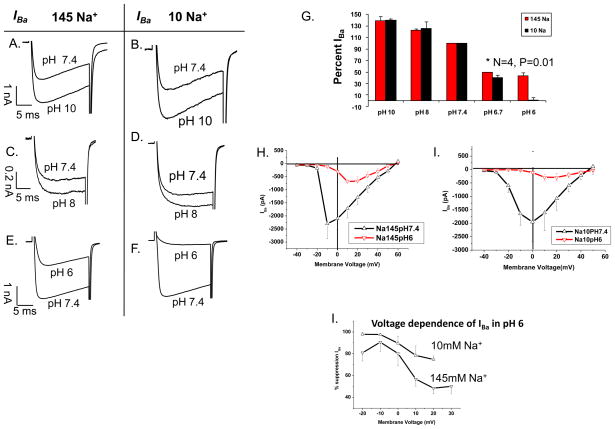
Under physiological conditions, an increase in extracellular proton concentrations has the strongest suppressive effects on the Ca2+ channel amongst the monovalents cations. We therefore tested if the effects of low Na+ might be influenced by acidification in a manner suggesting a common regulatory mechanism. Figure 9 compares the effect of changes in pH from 7.4 to 10, 8, and 6 in 145 (panels A,C,E) and 10 mM Na+ (panels B,D,F) on IBa measured in HEK cells expressing the recombinant L-type Ca2+ channel. Changing extracellular pH from 7.4 to 8 or 10 produced proportional enhancements of the current irrespective of the Na+ concentration (145 or 10 mM). However, at pH 6.7 there was a significant difference in the degree of suppression of IBa depending on external Na+ concentrations (50% in 145 and 60% in 10 mM Na+, Fig. 9G). At pH 6 the Na+ dependent suppressive effect of protons was even more pronounced (67% in 145 and 99% in 10 mM Na+, Fig. 9, EFG). The I-V relations in panels 9 H (145 mM Na+) and 9 I (10 mM Na+) show that such low pH values both reduce IBa and shift the V0.5 of activation parameter to more positive values. The voltage-dependence of the suppression of the Ca2+ channel current at pH 6 suggests that near complete block of the current may be anticipated at negative potentials, in 10 mM Na+ concentrations (Fig. 9J), but the block was mostly relieved at positive voltages, particularly in 145 mM Na+.
Figure 9. The effect of low extracellular [Na+] on pH sensitivity of IBa.
Panels A–F show Ba2+ current traces measured in the presence of 145 (A, C, E) and 10 mM (B, D, F) extracellular Na+ in individual cells where the response to low Na+ at pH 7.4 was compared that at either pH 10 (A–B), 8 (C–D), or 6 (E–F) G) Percent IBa compared to the current magnitude at pH 7.4 in response to varying extracellular pH values. H) I-V relation of IBa in 145mM external Na+ and pH 7.4 (△) or pH 6.0 (▽) (N=4). I) I-V relation of IBa in 10 mM external Na+ and pH 7.4 (△) or pH 6.0 (▽) (N=4). L) Voltage-dependence of IBa suppression in pH 6 and high or low external Na+ (N=3–4).
4. Discussion
This study provides novel insight into regulation of ICa by external Na+. We find that the reduction in current through the Ca2+ channel in Na+-deficient solutions is: a) rapid (5–15s), reversible (Figs. 1A, 2A, 3BCD, 4A, 7A, 8A), and dose-dependent (at a range relevant to clinical hyponatremia, 145–120 mM [Na+]o; Figs. 3, 6E, 7), b) independent of phosphorylation state of the channel (Fig. 2), c) somewhat dependent on the species of substituting monovalents (Li+<. Cs+ < TEA; Figs. 4, 5, 6E), the permeating divalent (Ca2+ vs. Ba2+), and the cellular environment of the native myocyte vs. heterologously expressed channel in HEK cells (Figs. 1–5, 7, 8 vs. 6, 9), but d) strongly modulated by acidification and agents that enhance the mean-open-time of the channel (Bay K, and Ba2+, Figs. 7 and 8).
Our results support the idea that Na+ interacts directly with the Ca2+ channel. We propose that Na+ may penetrate into the outer opening of the channel and occupy, in part, site(s) that are modulated by protons. This assessment is based on the evidence provided against direct Na+ permeation and CDI of the channel by influx of Ca2+ on NCX or CICR and on our new data in conjunction with published single-channel measurements, structural information, mutation analyses, and modeling efforts [29].
4.1. The Permeation Hypothesis Revisited
The primary evidence supporting direct permeation of Na+ through the Ca2+ channel was the report of a 8–13 mV negative shift in ICa reversal potential when extracellular Na+ was reduced by 50% [4]. Later studies however, did not support this possibility because the high Ca2+ selectivity (1 μM) of the channel precludes penetration of Na+ through the channels in presence of Ca2+ [3, 12–13]. In addressing the permeation possibility, we examined the change in the apparent reversal potential as a function of the substituting monovalents and their concentrations and found that shifts occurred only when Cs+ was substituted for Na+ (especially if Ba2+ was the charge carrier), while the suppression of ICa with Li+ or TEA+ had no significant effects on ICa reversal potential (Fig. 5). Cs+, however, produced no additional change in the reversal potential when substituting for [Na+]o below 100 mM concentrations (Cf. Fig. 3) or when Cs+ was used in a variable mixture with sucrose at a fixed [Na+]o of 50 mM (Cf. Fig. 4). The observed shifts in reversal potential (and activation curve; Fig. 7D) may be, in part, related to estimated −5 mV liquid junction potentials for Cs+. In short, our data do not support a correlation between the [Na+]o-induced changes in the magnitude and reversal potential of the current through the Ca2+ channel.
Considering the dose-response relation (Fig. 3), we note that lowering Na+ incrementally from 145-10 mM leads to a non-linear suppression of ICa, such that decreasing the Na+ concentrations from 145-70 mM Na+ decreased ICa in a concentration dependent manner, while from 70 to 10 mM the current was virtually independent of [Na+]o both in cardiomyocytes and the HEK cell expression system (Fig. 10A). Specifically, while a 25 mM reduction in Na+ from 145-120 mM suppressed ICa in ventricular cells by 20%, a 14 fold decrease from 70 to 5 mM suppressed the current by only another 13%. In contrast, if Na+ were permeating together with Ca2+ through the channel, then IC50 of the concentration-dependent suppression more likely would have been around 70 mM Na+, and not the measured 128 mM. We therefore conclude that permeation cannot contribute significantly to the low Na+-induced suppression of ICa.
Figure 10. Summary and analysis of the suppression of the Ca2+ channel by low Na+ and pH.
A) Dose-response of [Na+]o vs.: ICa measured with Cs+ substitution in ventricular cells (black trace and symbols, cf. Fig. 3A), IBa measured with sucrose substitution in the HEK cell expression system (red, cf. Fig. 6E), and IBa measured with Cs+ substitution in ventricular (green, cf.Fig. 7E). Solid curves are proportional to each other while the green dashed and dotted curves are cubic and linear in [Na+]o, respectively. B) Model suggesting different degrees of penetration of cations into (Na+, H+) and through (Ca2+, Ba2+) the Ca2+ channel where carboxylates (Y) may provide a negatively charged environment with binding sites both within the selectivity filter (EEEE locus) and in the outer vestibule. C) Combined effects of [Na+]o and pH on IBa based on Fig. 9G, but scaled to show the average response to low Na+ at pH 7.4. The data at different pH values were approximated with curves based on the logistic equation (A + B/(1+([H+]/KD)n)) showing no significant change to the effective binding constant in at 145 (black, circle, KD = 69±12 nM) vs. 10 mM [Na+]o (red, circle, KD = 90±8 nM). D) Conductance of the Ca2+ channel at different [Na+]o and pH measured from the linear segment of I-V relations (~10–50 mV, cf. Fig. 9HI). E) pH- and Na+-induced shifts in V0.5 of activation relative to V0.5 at pH 7.4 and 145 mM [Na+]o (Cf. Fig. 9H,I).
4.2. Role of NCX in Ca2+-dependent inactivation of ICa
The low Na+ suppression of ICa may also be mediated by CDI of the channel, secondary to influx of Ca2+ on NCX [3]. In physiological solutions (145 mM Na+) and free cytosolic Ca2+ of 100 nM, NCX operates primarily in the Ca2+ influx mode at voltages positive to −30 mV. In 10 mM Na+ solutions, however, ENa/Ca would shift to highly negative voltages of about −200 mV, greatly increasing the influx of Ca2+ on NCX, suppressing thereby ICa via the CDI mechanism. To circumvent this possibility cells were dialyzed with Na+-free solutions, which shifts ENa/Ca to positive potentials, counteracting the low external Na+-induced negative shift of ENa/Ca and minimizing Ca2+ entry on NCX. Since our results continued to show low Na+-induced ICa suppression even in zero Na+ dialyzed cells, we considered this mechanism improbable. This was also consistent with the findings that when Ba2+ was used as the charge carrier through the channel to prevent Ca2+ release from the SR [25], the suppressive effects of low Na+ on the Ca+2 channel were not significantly altered (Fig. 7).
4.3. Mechanism of Regulation of the Ca2+ Channel by Extracellular Na+
Although direct channel modulation by external Na+ has been considered [5], little evidence exists in its support. Several experimental approaches in the present study were directed at probing such a mechanism. Though the detailed structure of the pore-forming α1-subunit is still unknown [15], it is recognized that mutation of 4 centrally located glutamate residues (Fig. 10B; EEEE) strongly affects high-affinity Ca2+ binding, selectivity, threshold of activation, and pH-sensitivity of the channel [15, 29–30] thereby accounting for some of the major differences between high and low threshold Ca2+ channels, as well as the Na+ channel. The outer vestibule of the channel is also populated by acidic residues that may also modulate the conductance of the channel by establishing a negatively charged environment where cations can either bind to specific sites [31] or compete for the available space based on size, charge, and ability to incorporate carboxylate oxygens in their hydration shells [32].
This framework may be considered in the interpretation of our results. For instance, the efficacy of the substituting ions maybe related to their hydrated radii (Li+ < Cs+ < TEA+), which in turn would govern their penetration into the outer reaches of the channel pore. Permeation of Na+ to the selectivity filter is unlikely considering that monovalent conductance by the channel is abolished by μ-molar concentrations of Ca2+ [33]. The non-linear dose-response curves, showed no indication of saturation at [Na+]o of 145 mM and was not significantly altered by higher (155 mM) Na+ concentrations (Fig. 3A). In fact, irrespective of experimental condition, the effect on the Ca2+ channel current was in excess of cubed function of [Na+]o (Fig. 10A, dashed curve) suggesting a high degree of cooperativity. The multiple acidic residues in channel pore may favor high-cooperativity, but considering the substantial residual conductance (~55–70% at pH 7.4 and 10 mM Na+), it is also likely that a Na+-dependent linear permeation process (Fig. 10A; dotted line) is partially masked by a parallel Na+-independent permeation process. Figure 10C illustrates the combined effects of H+ and Na+ where the currents are all normalized relative to the value at pH 7.4 and 145 mM Na+ (Cf. Fig. 9G). Plotted this way it appears, that the IC50 of pH-dependence of the current is nearly independent of [Na+]o while the Na+-dependent component of the current is nearly the same at all pH values. This may be compared to the finding that acidification reduces the single Ca2+channel conductance from 140 to 45 pS [15, 34]. To distinguish more clearly between the effects of Na+ and H+, we analyzed the I-V relations (Fig. 8H, I) in terms of conductance and V0.5 of the activation parameter. The Na+-sensitive component of the whole-cell conductance amounted to ~30 nS and was insensitive to pH compared to the remaining conductance that changed gradually in the entire range from pH 6 to 10 (Fig. 10D). Similarly, the small Na+-dependent shift in the activation curves was independent of pH, but superimposed on a much larger pH-dependent shift at pH 6.0, which may account for the high overall pH-sensitivity of ICa in the narrower range of normal and slightly acidic conditions (Fig. 10E; Cf. [35]). Since mutation of the domain I and III glutamates of the EEEE locus abolishes the high-conductance state and renders the channel insensitive to pH [15], it would be of interest to test its sensitivity to Na+ under these conditions. In summary, our results are consistent with the notion that Na+ and H+ penetrate into the opening of the channel where they exert parallel, but antagonistic effects on channel conductance. Ultimately these effects may involve the selectivity filter, but it is as yet unclear whether the sensing of Na+ and H+ occurs at the same site. Similar considerations apply to our results testing phosphorylation (Fig. 2) and (S)-(−)Bay K 8644 (Fig. 8). Both interventions are known to enhance long openings of the Ca2+ channel [12, 36], but the mechanism would appear to be different since (S)-(−)-Bay K 8644, and dihydropyridines in general, are thought to penetrate into the outer vestibule [37–38], but PKA-dependent phosphorylation does not appear to directly target residues within the permeation pore [39]. Therefore, our findings that (S)-(−)-Bay K 8644 (Fig. 8), but not cAMP (Fig. 2) enhance the Na+-sensitivity of the channel are also consistent with the notion that Na+ interacts directly with the regulatory sites within the permeation pore.
5. Pathophysiological relevance
Hyponatremia is a clinical condition that results from a decline of serum Na+ levels below 134 mM. Duration and severity of hyponatremia is a strong predictor of clinical outcomes in many conditions including heart failure and myocardial infarction. It has long been known that patients with heart failure who also suffer from chronic hyponatremia have poor prognostic outcomes and have an increased rate of mortality [1]. Additionally patients with serum Na+ levels below 136 mM have an increased risk of developing heart failure and mortality post myocardial infarction [2, 40]. To date, no studies have mechanistically explored the link between hyponatremia and depressed cardiac contractility. Our studies here show that replacement of extracellular Na+ by other cations or sucrose directly suppressed L-type Ca2+ current in a concentration dependent manner with an IC50 of 128 mM (Figure 3). Since suppression of ICa in low Na+ is independent of channel phosphorylation, Ca2+-induced Ca2+ release, activation of Ca2+ influx mode of NCX, and is present in recombinant channel expressed in HEK cells, it is likely that it is a direct property of the channel itself. Na+ concentrations between 100–135 mM are concordant with serum Na+ levels associated with symptomatic hyponatremia. Decreased Ca2+ conductance with decreased external Na+ may in part explain the depressed cardiac contractility and poor outcomes encountered by patients suffering from persistent hyponatremia and heart failure especially under systemic or local acidosis commonly seen with ischemic injury.
Acknowledgments
This work was supported by NIH grants HL-16152 and 5 F32HL088855.
Abbreviations
- NCX
Na+/Ca2+ exchanger
- CICR
Ca2+-induced Ca2+ release
- CDI
Ca2+-dependent inactivation
- PKA
protein kinase A
- GFP
green fluorescent protein
- SR
sarcoplasmic reticulum
- I-V
current-voltage
Footnotes
Disclosures: None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brandimarte F, Fedele F, De Luca L, Fonarow GC, Gheorghiade M. Hyponatremia in acute heart failure syndromes: a potential therapeutic target. Curr Heart Fail Rep. 2007;4:207–213. doi: 10.1007/s11897-007-0014-5. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg A, Hammerman H, Petcherski S, et al. Hyponatremia and long-term mortality in survivors of acute ST-elevation myocardial infarction. Arch Intern Med. 2006;166:781–786. doi: 10.1001/archinte.166.7.781. [DOI] [PubMed] [Google Scholar]
- 3.Matsuda H, Noma A. Isolation of calcium current and its sensitivity to monovalent cations in dialysed ventricular cells of guinea-pig. J Physiol. 1984;357:553–573. doi: 10.1113/jphysiol.1984.sp015517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reuter H, Scholz H. A study of the ion selectivity and the kinetic properties of the calcium dependent slow inward current in mammalian cardiac muscle. J Physiol. 1977;264:17–47. doi: 10.1113/jphysiol.1977.sp011656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald TF, Pelzer S, Trautwein W, Pelzer DJ. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- 6.Noma A, Yanagihara K, Irisawa H. Inward current of the rabbit sinoatrial node cell. Pflugers Arch. 1977;372:43–51. doi: 10.1007/BF00582205. [DOI] [PubMed] [Google Scholar]
- 7.Bonvallet R, Rougier O. The effects of sodium removal on the two types of calcium currents in single frog atrial cells. Gen Physiol Biophys. 1991;10:523–536. [PubMed] [Google Scholar]
- 8.Mitchell MR, Powell T, Terrar DA, Twist VW. Characteristics of the second inward current in cells isolated from rat ventricular muscle. Proc R Soc Lond B Biol Sci. 1983;219:447–469. doi: 10.1098/rspb.1983.0084. [DOI] [PubMed] [Google Scholar]
- 9.Hume JR, Giles W. Ionic currents in single isolated bullfrog atrial cells. J Gen Physiol. 1983;81:153–194. doi: 10.1085/jgp.81.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nilius B, Hess P, Lansman JB, Tsien RW. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985;316:443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- 11.Campbell DL, Giles WR, Shibata EF. Ion transfer characteristics of the calcium current in bull-frog atrial myocytes. J Physiol. 1988;403:239–266. doi: 10.1113/jphysiol.1988.sp017248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hess P, Tsien RW. Mechanism of ion permeation through calcium channels. Nature. 1984;309:453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- 13.Almers W, McCleskey EW, Palade PT. A non-selective cation conductance in frog muscle membrane blocked by micromolar external calcium ions. J Physiol. 1984;353:565–583. doi: 10.1113/jphysiol.1984.sp015351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santarelli VP, Eastwood AL, Dougherty DA, Ahern CA, Horn R. Calcium block of single sodium channels: role of a pore-lining aromatic residue. Biophys J. 2007;93:2341–2349. doi: 10.1529/biophysj.107.106856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen XH, Bezprozvanny I, Tsien RW. Molecular basis of proton block of L-type Ca2+ channels. J Gen Physiol. 1996;108:363–374. doi: 10.1085/jgp.108.5.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pietrobon D, Prod’hom B, Hess P. Interactions of protons with single open L-type calcium channels. pH dependence of proton-induced current fluctuations with Cs+, K+, and Na+ as permeant ions. J Gen Physiol. 1989;94:1–21. doi: 10.1085/jgp.94.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knollmann BC, Knollmann-Ritschel BE, Weissman NJ, Jones LR, Morad M. Remodelling of ionic currents in hypertrophied and failing hearts of transgenic mice overexpressing calsequestrin. J Physiol. 2000;525(Pt 2):483–498. doi: 10.1111/j.1469-7793.2000.t01-1-00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belmonte S, Morad M. ‘Pressure-flow’-triggered intracellular Ca2+ transients in rat cardiac myocytes: possible mechanisms and role of mitochondria. J Physiol. 2008;586:1379–1397. doi: 10.1113/jphysiol.2007.149294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reuter H, Scholz H. The regulation of the calcium conductance of cardiac muscle by adrenaline. J Physiol. 1977;264:49–62. doi: 10.1113/jphysiol.1977.sp011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 21.Douglas L, Davies A, Wratten J, Dolphin AC. Do voltage-gated calcium channel alpha2delta subunits require proteolytic processing into alpha2 and delta to be functional? Biochem Soc Trans. 2006;34:894–898. doi: 10.1042/BST0340894. [DOI] [PubMed] [Google Scholar]
- 22.Canti C, Nieto-Rostro M, Foucault I, et al. The metal-ion-dependent adhesion site in the Von Willebrand factor-A domain of alpha2delta subunits is key to trafficking voltage-gated Ca2+ channels. Proc Natl Acad Sci U S A. 2005;102:11230–11235. doi: 10.1073/pnas.0504183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sham JS, Cleemann L, Morad M. Functional coupling of Ca2+ channels and ryanodine receptors in cardiac myocytes. Proc Natl Acad Sci U S A. 1995;92:121–125. doi: 10.1073/pnas.92.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adachi-Akahane S, Cleemann L, Morad M. Cross-signaling between L-type Ca2+ channels and ryanodine receptors in rat ventricular myocytes. J Gen Physiol. 1996;108:435–454. doi: 10.1085/jgp.108.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nabauer M, Callewaert G, Cleemann L, Morad M. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science. 1989;244:800–803. doi: 10.1126/science.2543067. [DOI] [PubMed] [Google Scholar]
- 26.Mentrard D, Vassort G, Fischmeister R. Calcium-mediated inactivation of the calcium conductance in cesium-loaded frog heart cells. J Gen Physiol. 1984;83:105–131. doi: 10.1085/jgp.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hess P, Lansman JB, Tsien RW. Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells. J Gen Physiol. 1986;88:293–319. doi: 10.1085/jgp.88.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adachi-Akahane S, Cleemann L, Morad M. BAY K 8644 modifies Ca2+ cross signaling between DHP and ryanodine receptors in rat ventricular myocytes. Am J Physiol. 1999;276:H1178–1189. doi: 10.1152/ajpheart.1999.276.4.H1178. [DOI] [PubMed] [Google Scholar]
- 29.Sather WA, McCleskey EW. Permeation and selectivity in calcium channels. Annu Rev Physiol. 2003;65:133–159. doi: 10.1146/annurev.physiol.65.092101.142345. [DOI] [PubMed] [Google Scholar]
- 30.Talavera K, Staes M, Janssens A, et al. Aspartate residues of the Glu-Glu-Asp-Asp (EEDD) pore locus control selectivity and permeation of the T-type Ca(2+) channel alpha(1G) J Biol Chem. 2001;276:45628–45635. doi: 10.1074/jbc.M103047200. [DOI] [PubMed] [Google Scholar]
- 31.Feng ZP, Hamid J, Doering C, et al. Amino acid residues outside of the pore region contribute to N-type calcium channel permeation. J Biol Chem. 2001;276:5726–5730. doi: 10.1074/jbc.C000791200. [DOI] [PubMed] [Google Scholar]
- 32.Nonner W, Eisenberg B. Ion permeation and glutamate residues linked by Poisson-Nernst-Planck theory in L-type calcium channels. Biophys J. 1998;75:1287–1305. doi: 10.1016/S0006-3495(98)74048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang J, Ellinor PT, Sather WA, Zhang JF, Tsien RW. Molecular determinants of Ca2+ selectivity and ion permeation in L-type Ca2+ channels. Nature. 1993;366:158–161. doi: 10.1038/366158a0. [DOI] [PubMed] [Google Scholar]
- 34.Prod’hom B, Pietrobon D, Hess P. Interactions of protons with single open L-type calcium channels. Location of protonation site and dependence of proton-induced current fluctuations on concentration and species of permeant ion. J Gen Physiol. 1989;94:23–42. doi: 10.1085/jgp.94.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delisle BP, Satin J. pH modification of human T-type calcium channel gating. Biophys J. 2000;78:1895–1905. doi: 10.1016/S0006-3495(00)76738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ochi R. Single-channel mechanism of beta-adrenergic enhancement of cardiac L-type calcium current. Jpn J Physiol. 1993;43:571–584. doi: 10.2170/jjphysiol.43.571. [DOI] [PubMed] [Google Scholar]
- 37.Kwan YW, Bangalore R, Lakitsh M, Glossmann H, Kass RS. Inhibition of cardiac L-type calcium channels by quaternary amlodipine: implications for pharmacokinetics and access to dihydropyridine binding site. J Mol Cell Cardiol. 1995;27:253–262. doi: 10.1016/s0022-2828(08)80024-7. [DOI] [PubMed] [Google Scholar]
- 38.Tikhonov DB, Zhorov BS. Structural model for dihydropyridine binding to L-type calcium channels. J Biol Chem. 2009;284:19006–19017. doi: 10.1074/jbc.M109.011296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erxleben C, Gomez-Alegria C, Darden T, Mori Y, Birnbaumer L, Armstrong DL. Modulation of cardiac Ca(V)1.2 channels by dihydropyridine and phosphatase inhibitor requires Ser-1142 in the domain III pore loop. Proc Natl Acad Sci U S A. 2003;100:2929–2934. doi: 10.1073/pnas.2628046100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singla I, Zahid M, Good CB, Macioce A, Sonel AF. Effect of hyponatremia (<135 mEq/L) on outcome in patients with non-ST-elevation acute coronary syndrome. Am J Cardiol. 2007;100:406–408. doi: 10.1016/j.amjcard.2007.03.039. [DOI] [PubMed] [Google Scholar]