Abstract
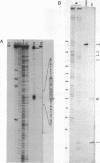
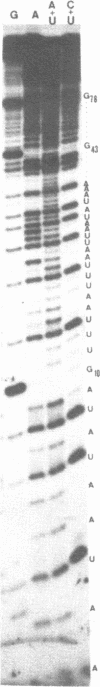
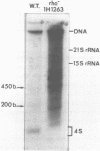
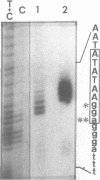
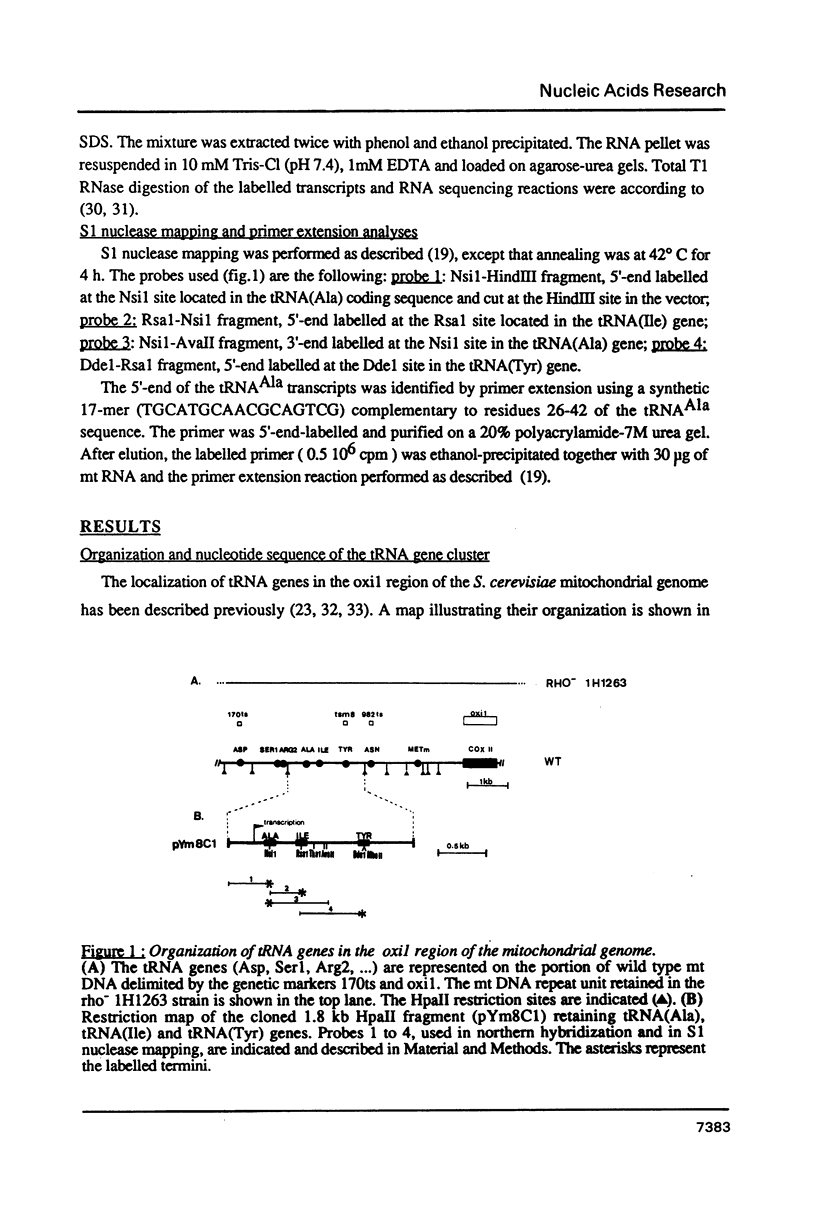
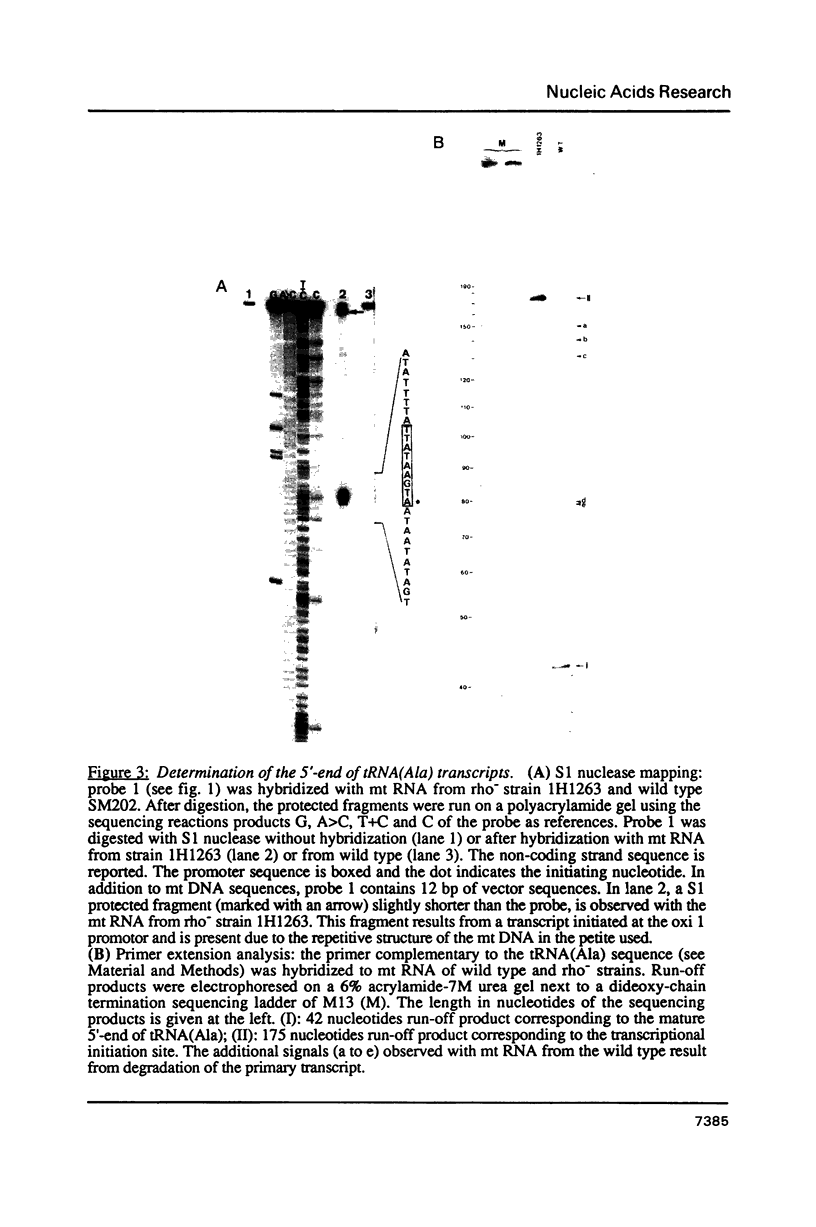
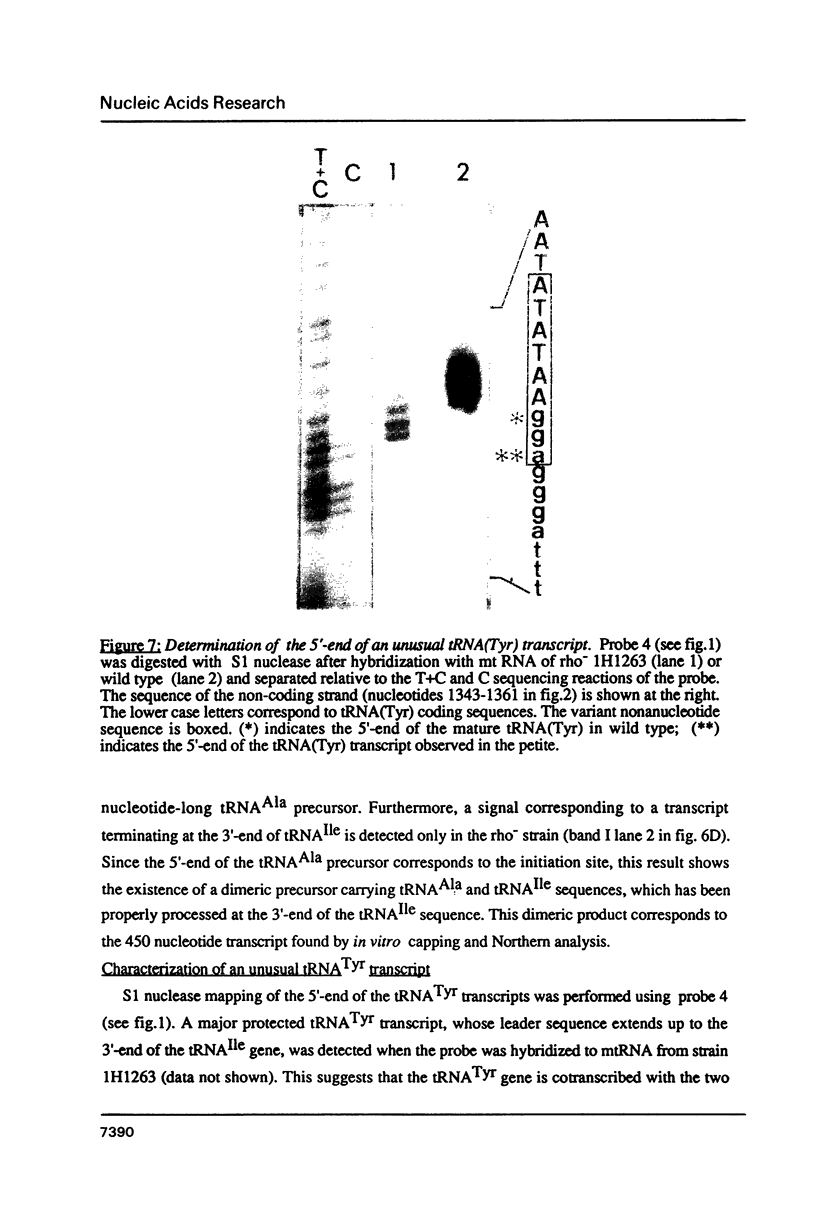
Expression of 5 yeast mitochondrial tRNA genes (Ala, Ile, Tyr, Asn and Metm), localized upstream from the oxil gene has been analyzed by in vitro capping using guanylyltransferase, northern hybridization and S1 nuclease mapping in the wild type and a rho-strain. The 5 tRNA sequences belong to the same transcriptional unit which is initiated 133 bp upstream from the tRNA(Ala) gene at a promoter sequence TTATAAGTA. Furthermore, a truncated tRNA(Tyr) transcript, 2 nucleotides shorter than mature tRNA(Tyr) has been found, only in the rho-strain. This minor transcript may result from secondary transcription initiation at a variant nonanucleotide sequence, ATATAAGGA, which overlaps the tRNA(Tyr) coding sequence by 3 nucleotides. The polycistronic precursor has proven to be useful in investigation of the mechanisms of tRNA processing. Maturation of this primary transcript proceeds exclusively by precise endonucleolytic cleavages at the 5' and 3'-ends of tRNA sequences.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechmann H., Krüger M., Böker E., Bandlow W., Schweyen R. J., Kaudewitz F. On the formation of rho- petites in yeast. II. Effects of mutation tsm-8 on mitochondrial functions and rho-factor stability in Saccharomyces cerevisiae. Mol Gen Genet. 1977 Sep 21;155(1):41–51. doi: 10.1007/BF00268559. [DOI] [PubMed] [Google Scholar]
- Biswas T. K., Edwards J. C., Rabinowitz M., Getz G. S. Characterization of a yeast mitochondrial promoter by deletion mutagenesis. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1954–1958. doi: 10.1073/pnas.82.7.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas T. K., Getz G. S. A critical base in the yeast mitochondrial nonanucleotide promoter. Abolition of promoter activity by mutation at the -2 position. J Biol Chem. 1986 Mar 25;261(9):3927–3930. [PubMed] [Google Scholar]
- Biswas T. K., Getz G. S. Nucleotides flanking the promoter sequence influence the transcription of the yeast mitochondrial gene coding for ATPase subunit 9. Proc Natl Acad Sci U S A. 1986 Jan;83(2):270–274. doi: 10.1073/pnas.83.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonitz S. G., Tzagoloff A. Assembly of the mitochondrial membrane system. Sequences of yeast mitochondrial tRNA genes. J Biol Chem. 1980 Oct 10;255(19):9075–9081. [PubMed] [Google Scholar]
- Bordonné R., Bandlow W., Dirheimer G., Martin R. P. A single base change in the extra-arm of yeast mitochondrial tyrosine tRNA affects its conformational stability and impairs aminoacylation. Mol Gen Genet. 1987 Mar;206(3):498–504. doi: 10.1007/BF00428891. [DOI] [PubMed] [Google Scholar]
- Castaño J. G., Tobian J. A., Zasloff M. Purification and characterization of an endonuclease from Xenopus laevis ovaries which accurately processes the 3' terminus of human pre-tRNA-Met(i) (3' pre-tRNase). J Biol Chem. 1985 Jul 25;260(15):9002–9008. [PubMed] [Google Scholar]
- Christianson T., Edwards J. C., Mueller D. M., Rabinowitz M. Identification of a single transcriptional initiation site for the glutamic tRNA and COB genes in yeast mitochondria. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5564–5568. doi: 10.1073/pnas.80.18.5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson T., Rabinowitz M. Identification of multiple transcriptional initiation sites on the yeast mitochondrial genome by in vitro capping with guanylyltransferase. J Biol Chem. 1983 Nov 25;258(22):14025–14033. [PubMed] [Google Scholar]
- Coruzzi G., Tzagoloff A. Assembly of the mitochondrial membrane system. DNA sequence of subunit 2 of yeast cytochrome oxidase. J Biol Chem. 1979 Sep 25;254(18):9324–9330. [PubMed] [Google Scholar]
- Deutscher M. P. Processing of tRNA in prokaryotes and eukaryotes. CRC Crit Rev Biochem. 1984;17(1):45–71. doi: 10.3109/10409238409110269. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. C., Levens D., Rabinowitz M. Analysis of transcriptional initiation of yeast mitochondrial DNA in a homologous in vitro transcription system. Cell. 1982 Dec;31(2 Pt 1):337–346. doi: 10.1016/0092-8674(82)90127-1. [DOI] [PubMed] [Google Scholar]
- Edwards J. C., Osinga K. A., Christianson T., Hensgens L. A., Janssens P. M., Rabinowitz M., Tabak H. F. Initiation of transcription of the yeast mitochondrial gene coding for ATPase subunit 9. Nucleic Acids Res. 1983 Dec 10;11(23):8269–8282. doi: 10.1093/nar/11.23.8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelke D. R., Gegenheimer P., Abelson J. Nucleolytic processing of a tRNAArg-tRNAAsp dimeric precursor by a homologous component from Saccharomyces cerevisiae. J Biol Chem. 1985 Jan 25;260(2):1271–1279. [PubMed] [Google Scholar]
- Frendewey D., Dingermann T., Cooley L., Söll D. Processing of precursor tRNAs in Drosophila. Processing of the 3' end involves an endonucleolytic cleavage and occurs after 5' end maturation. J Biol Chem. 1985 Jan 10;260(1):449–454. [PubMed] [Google Scholar]
- Frontali L., Palleschi C., Francisci S. Transcripts of mitochondrial tRNA genes in Saccharomyces cerevisiae. Nucleic Acids Res. 1982 Nov 25;10(22):7283–7293. doi: 10.1093/nar/10.22.7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrier-Takada C., Gardiner K., Marsh T., Pace N., Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983 Dec;35(3 Pt 2):849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- Hollingsworth M. J., Martin N. C. RNase P activity in the mitochondria of Saccharomyces cerevisiae depends on both mitochondrion and nucleus-encoded components. Mol Cell Biol. 1986 Apr;6(4):1058–1064. doi: 10.1128/mcb.6.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. L., Greenleaf A. L., Lehman I. R. Isolation of the nuclear gene encoding a subunit of the yeast mitochondrial RNA polymerase. J Biol Chem. 1986 Aug 5;261(22):10348–10351. [PubMed] [Google Scholar]
- Kelly J. L., Lehman I. R. Yeast mitochondrial RNA polymerase. Purification and properties of the catalytic subunit. J Biol Chem. 1986 Aug 5;261(22):10340–10347. [PubMed] [Google Scholar]
- Levens D., Ticho B., Ackerman E., Rabinowitz M. Transcriptional initiation and 5' termini of yeast mitochondrial RNA. J Biol Chem. 1981 May 25;256(10):5226–5232. [PubMed] [Google Scholar]
- Locker J. Analytical and preparative electrophoresis of RNA in agarose-urea. Anal Biochem. 1979 Oct 1;98(2):358–367. doi: 10.1016/0003-2697(79)90154-4. [DOI] [PubMed] [Google Scholar]
- Locker J., Rabinowitz M. Transcription in yeast mitochondria: analysis of the 21 S rRNA region and its transcripts. Plasmid. 1981 Nov;6(3):302–314. doi: 10.1016/0147-619x(81)90038-x. [DOI] [PubMed] [Google Scholar]
- Martin N. C., Miller D. L., Underbrink K., Ming X. Structure of a precursor to the yeast mitochondrial tRNAMetf. Implications for the function of the tRNA synthesis locus. J Biol Chem. 1985 Feb 10;260(3):1479–1483. [PubMed] [Google Scholar]
- Martin N. C., Underbrink-Lyon K. A mitochondrial locus is necessary for the synthesis of mitochondrial tRNA in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4743–4747. doi: 10.1073/pnas.78.8.4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Martin N. C. Characterization of the yeast mitochondrial locus necessary for tRNA biosynthesis: DNA sequence analysis and identification of a new transcript. Cell. 1983 Oct;34(3):911–917. doi: 10.1016/0092-8674(83)90548-2. [DOI] [PubMed] [Google Scholar]
- Morimoto R., Locker J., Synenki R. M., Rabinowitz M. Transcription, processing, and mapping of mitochondrial RNA from grande and petite yeast. J Biol Chem. 1979 Dec 25;254(24):12461–12470. [PubMed] [Google Scholar]
- Newman D., Pham H. D., Underbrink-Lyon K., Martin N. C. Characterization of tRNA genes in tRNA region II of yeast mitochondrial DNA. Nucleic Acids Res. 1980 Nov 11;8(21):5007–5016. doi: 10.1093/nar/8.21.5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nóbrega M. P., Nóbrega F. G. Mapping and sequencing of the wild-type and mutant (G116-40) alleles of the tyrosyl-tRNA mitochondrial gene in Saccharomyces cerevisiae. J Biol Chem. 1986 Mar 5;261(7):3054–3059. [PubMed] [Google Scholar]
- Osinga K. A., De Vries E., Van der Horst G. T., Tabak H. F. Initiation of transcription in yeast mitochondria: analysis of origins of replication and of genes coding for a messenger RNA and a transfer RNA. Nucleic Acids Res. 1984 Feb 24;12(4):1889–1900. doi: 10.1093/nar/12.4.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palleschi C., Francisci S., Bianchi M. M., Frontali L. Initiation of transcription of a mitochondrial tRNA gene cluster in S. cerevisiae. Nucleic Acids Res. 1984 Oct 11;12(19):7317–7326. doi: 10.1093/nar/12.19.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palleschi C., Francisci S., Zennaro E., Frontali L. Expression of the clustered mitochondrial tRNA genes in Saccharomyces cerevisiae: transcription and processing of transcripts. EMBO J. 1984 Jun;3(6):1389–1395. doi: 10.1002/j.1460-2075.1984.tb01982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich C., Gardiner K. J., Olsen G. J., Pace B., Marsh T. L., Pace N. R. The RNA component of the Bacillus subtilis RNase P. Sequence, activity, and partial secondary structure. J Biol Chem. 1986 Jun 15;261(17):7888–7893. [PubMed] [Google Scholar]
- Schinkel A. H., Groot Koerkamp M. J., Van der Horst G. T., Touw E. P., Osinga K. A., Van der Bliek A. M., Veeneman G. H., Van Boom J. H., Tabak H. F. Characterization of the promoter of the large ribosomal RNA gene in yeast mitochondria and separation of mitochondrial RNA polymerase into two different functional components. EMBO J. 1986 May;5(5):1041–1047. doi: 10.1002/j.1460-2075.1986.tb04320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibler A. P., Dirheimer G., Martin R. P. The primary structure of yeast mitochondrial tyrosine tRNA. FEBS Lett. 1983 Feb 21;152(2):153–156. doi: 10.1016/0014-5793(83)80368-8. [DOI] [PubMed] [Google Scholar]
- Sibler A. P., Dirheimer G., Martin R. P. Yeast mitochondrial tRNAIle and tRNAMetm: nucleotide sequence and codon recognition patterns. Nucleic Acids Res. 1985 Feb 25;13(4):1341–1345. doi: 10.1093/nar/13.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Hartmann T., Meissner F., Moll J., Vorderwülbecke T. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1987;15 (Suppl):r53–188. doi: 10.1093/nar/15.suppl.r53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowski M., Fukuhara H. The genetic map of transfer RNA genes of yeast mitochondria: correction and extension. Mol Gen Genet. 1979 Mar 5;170(3):261–275. doi: 10.1007/BF00267059. [DOI] [PubMed] [Google Scholar]
- Wettstein-Edwards J., Ticho B. S., Martin N. C., Najarian D., Getz G. S. In vitro transcription and promoter strength analysis of five mitochondrial tRNA promoters in yeast. J Biol Chem. 1986 Feb 25;261(6):2905–2911. [PubMed] [Google Scholar]
- Winkley C. S., Keller M. J., Jaehning J. A. A multicomponent mitochondrial RNA polymerase from Saccharomyces cerevisiae. J Biol Chem. 1985 Nov 15;260(26):14214–14223. [PubMed] [Google Scholar]
- Yousaf S. I., Carroll A. R., Clarke B. E. A new and improved method for 3'-end labelling DNA using [alpha-32P]ddATP. Gene. 1984 Mar;27(3):309–313. doi: 10.1016/0378-1119(84)90075-1. [DOI] [PubMed] [Google Scholar]