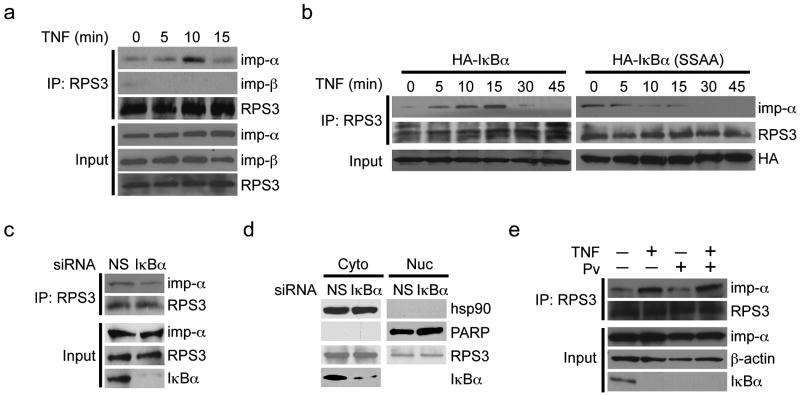
Figure 3. Importin-α-mediated nuclear translocation of RPS3 is IκBα degradation dependent.
(a) Whole-cell lysates (Input) from Jurkat cells stimulated as indicated were directly immunoblotted, or after immunoprecipitation (IP) with RPS3 antibody, for importin-α imp-α, importin-β imp-β, or RPS3. (b) Immunoprecipitation (IP)/immunoblot of the association of the endogenous importin-α imp-α or importin-β imp-β to RPS3 in Jurkat cells overexpressing either wild-type or SSAA mutant HA-IκBα and stimulated with TNF as indicated. (c) Jurkat cells were transfected with nonspecific (NS) or IκBα siRNA. 72 h later, whole-cell lysates (Input) were immunoblotted directly or after immunoprecipitation (IP) with RPS3 antibody for indicated proteins. (d) Immunoblotting of cytosolic (Cyto) and nuclear (Nuc) subcellular fractions derived from Jurkat cells transfected with scrambled nonspecific (NS), or IκBα siRNAs. Hsp90 and PARP served as cytosolic and nuclear markers and loading controls, respectively. (e) Jurkat cells were pretreated with (+) or without (-) sodium pervanadate (Pv, 800 mM) for 2 h followed by a 30-min TNF stimulation. Whole-cell lysates (Input) were immunoblotted directly or after immunoprecipitation (IP) with anti-RPS3 antibody for indicated proteins. Data are representative of at least of two experiments.