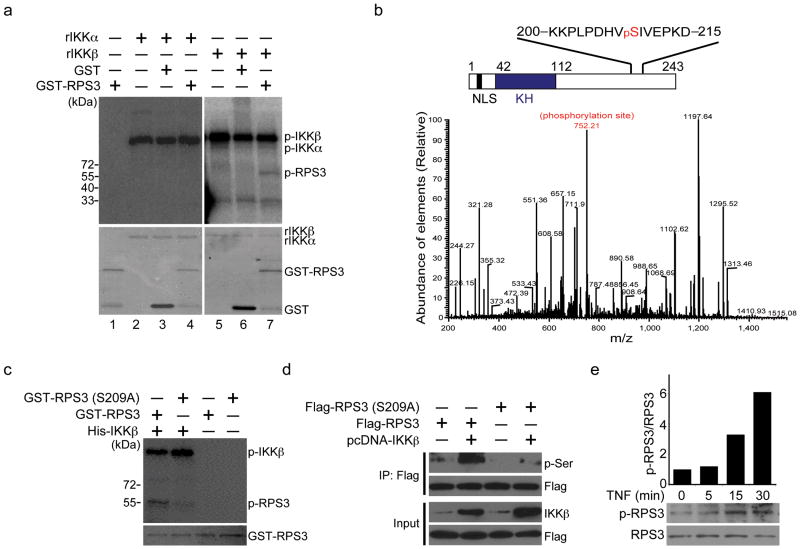
Figure 4. IKKβ phosphorylates RPS3 at serine 209.
(a) Autoradiograph (top) and Coomassie blue staining (bottom) of in vitro kinase assays performed with recombinant GST or GST-RPS3 protein using recombinant human IKKα (rIKKα) or IKKβ (rIKKβ) as kinases. The autophosphorylated IKKs (p-IKKα and p-IKKβ) and phosphorylated RPS3 (p-RPS3) are labeled, respectively. (b) In vitro kinase assays were performed using recombinant RPS3 protein with or without rIKKβ as kinases. After digestion, the phosphorylated peptides were enriched by TiO2 and fragmented by mass spectrometer. MS/MS of the 1+ fragment ion displays indicative of phosphorylated KKPLPDHVpSIVEPKD based on a Mascot algorithm database search. The y6 ion (in red) shows the incorporation of the site of phosphorylation, which is further confirmed by the loss of H3PO4 from several ions (bottom). Schematic diagram of RPS3 with characterized domains (NLS, nuclear localization signal; KH, K homology) and the IKKβ phosphorylation site serine 209 highlighted in red (top). (c) Autoradiograph (top) and Coomassie blue staining (bottom) of in vitro kinase assays performed with recombinant wild-type or S209A mutant GST-RPS3 proteins using recombinant human IKKβ. The phosphorylated (p-RPS3), total GST-RPS3 proteins, and autophosphorylated IKKβ (p-IKKβ) are labeled, respectively. (d) Immunoprecipitation (IP)/immunoblot of phosphoserine for ectopically expressed RPS3 in HEK 293T cells, where wild-type or S209A mutant Flag-RPS3 was transfected with or without an IKKβ plasmid. (e) Immunoblot of phospho-RPS3 (p-RPS3) and RPS3 in whole cell lysates derived from Jurkat cells stimulated with TNF for the indicated periods (bottom). Densitometry of all bands was performed, and the intensity of each p-RPS3 band was normalized to corresponding RPS3 band. The fold change in p-RPS3/RPS3 ratio was further normalized to the unstimulated sample (top). Data are representative of four (a, d), and two (b, c, d) independent experiments, respectively.