Abstract
The role of sensory acuity, processing speed, and working memory capacity on auditory working memory span (L-span) performance at five presentation levels was examined in 80 young (18–30 y) and 26 older adults (60–82 y). Lowering the presentation level of the L-span task had a greater detrimental effect on older adults than in the young. Furthermore, the relationship between sensory acuity and L-span performance varied as a function of age and presentation level. These results suggest that declining acuity plays an important explanatory role in age-related declines in cognitive abilities.
Keywords: auditory acuity, working memory, listening span, cognitive aging, perceptual processing, hearing
Age-related declines in cognitive skills are typically found for fluid intelligence (see reviews in Salthouse, 2003; Verhaeghen & Salthouse, 1997), prospective memory (Kliegel & Jager, 2006; Kliegel, Jager, & Phillips, 2008), episodic memory (Addis, Wong, & Schacter, 2008; Old & Naveh-Benjamin, 2008), executive processing (Kray, Eber, & Lindenberger, 2004) and dual task performance (Glass et al., 2000; Verhaeghen, Steitz, Sliwinski, & Cerella, 2003). Some researchers have proposed that such age related difficulties reflect impaired cognitive functions such as slowed information processing (Cerella, 1985; Salthouse, 1994) or decreased working memory/attentional capacity (Park et al., 2002; Salthouse, 1992). Others have pointed out, however, that age related declines in sensory acuity may lead to difficulties in performing cognitive tasks, even when visual or auditory stimuli are presented at above-threshold levels such that they can be seen or heard (Baldwin, 2002; Baldwin, 2007; Baldwin & Struckman-Johnson, 2002; Lunner, Rudner, & Ronnberg, 2009; Pichora-Fuller, Schneider, & Daneman, 1995; Schneider, Daneman, & Murphy, 2005; Wingfield, Tun, & McCoy, 2005). Accordingly, mediation of cognitive performance by sensory acuity provides another explanatory framework for age-related cognitive decline (see discussions in Baldwin, 2002; Rabbitt, 1990; Schneider et al., 2005; Schneider & Pichora-Fuller, 2000; Scialfa, 2002).
Hearing impairment is pervasive among older adults (Corso, 1963; Gates, Cooper, Kannel, & Miller, 1990). In a recent population based sample of over 2000 adults 73–84 years of age, 59.9% had mid frequency clinical hearing loss (pure-tone thresholds of > 25 dB at 500, 1000, and 2000 Hz) and 76.9% had high frequency hearing loss (Helzner et al., 2005). Sub-clinical hearing loss among older adults is ubiquitous. Pure-tone thresholds have consistently been found to increase with age (Corso, 1963; Fozard & Gordon-Salant, 2001; Schieber & Baldwin, 1996), particularly across the high frequency range which is critical for distinguishing between consonants (Botwinick, 1984; Humes, 2007; Villaume, Brown, & Darling, 1994).
Hearing impairment may impact higher order cognitive processes in several ways. First, it may result in the sensory extraction stage of speech processing requiring greater allocation of limited attentional resources, leaving fewer resources available for other processing tasks such as binding content with context (Bopp & Verhaeghen, 2009) and storing information for later recall (McCoy et al., 2005; Pichora-Fuller et al., 1995; Rabbitt, 1968, 1990; Sarampalis, Kalluri, Edwards, & Hafter, 2009; Wingfield et al., 2005). Reduced presentation level has been shown to negatively impact speech processing even among young listeners in attentionally demanding conditions (Baldwin & Struckman-Johnson, 2002). Secondly, hearing impairment, similar to reduced presentation level, may degrade both the quality and persistence of the echoic memory trace (Baldwin, 2007), a factor that may place older adults at particular disadvantage in difficult and/or dual task situations. Thirdly, hearing impairment may result in a greater number of irrelevant stimuli being generated during the recognition stages, which would subsequently require greater effort to ignore or discard. Accounting for acoustic-peripheral factors is therefore critical to ensuring that older adults are not disadvantaged, relative to young adults, in assessments of the impact of irrelevant stimuli on performance (Li, Daneman, Qi, & Schneider, 2004).
These three explanations for the impact of sensory impairment on cognitive processing are not mutually exclusive. In fact, each may contribute independently or interactively to explain why sensory impairment accounts for such a large amount of the age-related variance in cognitive performance in an increasingly wide range of tasks (Li et al., 2004; Lindenberger & Baltes, 1994). The need to consider the interacting affects of auditory and cognitive processes in speech understanding has gained increased attention in recent years (see Arlinger, Lunner, Lyxell, & Pichora-Fuller, 2009; Pichora-Fuller, 2007) and it has particular relevance to understanding the communication and performance difficulties of older adults (Baldwin, 2002). The aim of the current study was to expand the knowledge base in this important area.
In order to investigate the role that cognitive and sensory factors play in age related declines in cognitive task performance, we examined the contributions of working memory capacity as assessed visually (reading span, R-span), processing speed (Trail making test – part B, TMT-B), and sensory acuity (speech reception threshold, SRT) to the performance an auditory version of a working memory span task (listening span, L-span) in younger and older adults free from clinically significant hearing impairment. We manipulated the presentation intensity of the L-span stimuli at levels that were above the participants’ speech reception threshold in order to investigate the effects of sound level on cognitive task performance. Based on the theory that sensory acuity plays a central role in age related declines in cognitive performance, we made the following predictions: 1) decreasing presentation level at above threshold levels will result in reduced auditory working memory performance in both younger and older adults, 2) decreasing presentation level will have a greater impact on performance in older adults, 3) working memory capacity measured in a visually presented task and L-span performance will be the best predictor of auditory working memory span performance in younger adults, 4) speech reception threshold will be the best predictor of auditory working memory performance in older adults, and 5) the relationship between speech reception threshold and auditory working memory performance will increase as presentation level decreases in both younger and older adults.
Method
Participants
The young group consisted of 80 undergraduate students (aged 18–31 y, M = 20.6, SD = 2.76 y, 60 females) enrolled in psychology courses who voluntarily participated in this study for research credits. The older group consisted of 26 community dwelling older participants (aged 60–80 y, M = 68.6 y, SD = 6.2, 15 females) who voluntarily participated and received $20 for their time. All participants were currently licensed and active drivers, in self-reported good health, free of mild cognitive impairment and dementia as confirmed by scores of > 26 on the Mini-Mental State Exam (Folstein, Folstein, & McHugh, 1975) and were monolingual English speakers. All participants had a minimum of a high school diploma and the majority had completed at least two years of college. All participants had self-reported normal or corrected to normal vision and hearing. All participants also passed a pure-tone audiometric screening indicating they had < 26 dB hearing level at 500, 1000, 2000, 4000 and 8000 Hz while sitting in the sound attenuated experimental testing room and wearing the same headphones (with active noise cancelation on) used to present the L-span stimuli. Note that we specifically made efforts to recruit older adults that were free from known hearing impairment. Despite this recruitment effort, 9 individuals failed to pass the pure-tone screening and were thus eliminated from further experimental testing and not included in the older sample.
Materials
Reading Span (R-span) Task
The R-span task, was originally developed by Daneman and Carpenter (1983) and later modified by Engle, Kane and colleagues (Engle, Kane, & Tuholski, 1999; Kane, Bleckley, Conway, & Engle, 2001; Turner & Engle, 1989). Despite minor variation in its administration and scoring, the R-span task has been in widespread use for some time as an index of working memory capacity (for a methodological review see Conway et al., 2005) The modified version was used with sentence set lengths being presented in randomized order and total score reported as the proportion of words correctly recalled in the correct order averaged across each set size. R-span served as our measure of individual differences in working memory capacity.
Trail Making Test – part B (TMT-B)
The TMT-B is commonly administered in neuropsychological assessments (Salthouse et al., 2000). Participants pointed to alternating sequences of numbers and letters (i.e., 1, A, 2, B, 3, C etc...) as quickly as possible. Participants were corrected as quickly as possible if an error was made (1955 (1958). Validity analyses imply that Part B can be used in isolation from Part A and Part B provides a sensitive index of perceptual speed (de Frias, Dixon, Fisher, & Camicioli, 2007) though it is also used to assess set switching. TMT-B correlates moderately well with other tasks that are commonly used as measures of perceptual speed, such as the Digit Symbol Substitution Task and the Trail Making Task – Part A (Burton, Strauss, Hultsch, & Hunter, 2006; Salthouse, 1994). More than three errors are considered out of range performance and participants are allowed to discontinue the task. All participants in the current investigations were able to complete the TMT-B task with less than three errors. TMT-B served as our measure of individual differences in processing speed.
Speech Reception Threshold
The Speech Reception Threshold (SRT) assessment provides an index of the minimum threshold at which participants can recognize two-syllable Spondee words more than 50% of the time. Following guidelines established by the American Speech-Language-Hearing Association (ASHA, 1979), the experimenter first read a list of the to-be-presented spondee words in random order to each participant. Then digitized words were presented through Sony MDR-NC60 headphones with active noise cancelation turned on. Digitized words were spoken by a male whose first language was English speaking at a normal conversation level (averaging 62.5 dBA) from approximately six inches away from a Nady 24M USB microphone at a sample rate of 22050 Hz and 16 bit resolution. Digitized recordings were then amplified and attenuated using Cool Edit@ software to achieve the experimental presentation levels. Stimuli were normalized using root mean square (RMS) values to ensure consistency across stimuli at each level. Presentation levels were verified by obtaining a visual running average using a Bruel & Kjaer 2238 Mediator Sound Level Meter on the slow setting (see Tschopp, Beckenbauer, & Harris, 1991). A Pentium III computer running customized programs developed in Visual Basic were used to present all auditory stimuli. As specified in ASHA guidelines (ASHA, 1979; Newby, 1972), the words were presented in sets of four and increased by 3 decibels on an A weighted scale (dBA). Seven levels were used in the current experiment including 27, 30, 33, 36, 39, 42 and 45 dBA. Participants repeated out loud each word if they could hear it. SRT served as our measure of individual differences in auditory acuity.
Listening Span (L-span) Task
A modification of Daneman and Carpenter’s (1983) reading span task, called the listening span (L-span) (after Daneman & Carpenter, 1980) was presented. The L-span stimuli were recorded using the same procedures, equipment, same sample rate and resolution as the SRT, but were recorded by a female speaker. In this task, participants heard a series of short sentences that were either logical or illogical. After verifying the logic of each sentence by key press, participants were asked to recall the sentence final word from each sentence in the series. Initially, two sentences were presented followed by a 750 Hz tone lasting 200 ms at 60 dBA indicating that they were to recall aloud each of the sentence final words from the set. If they missed a word from the sentence set, the same size sentence set was repeated with a different list of sentences. If the participant accurately recalled all words from that set, the number of sentences included in the set increased by one. This procedure was repeated until the listener made recall errors on two consecutive presentations of a given set size. Series of sentences were presented at levels of 45, 50, 55, 60, and 65 dBA in counter balanced order. L-span score was defined as the sentence series length at which participants could correctly recall all sentence final words in any order. The differences between the administration and scoring procedure for the L-span and R-span tasks were due to the fact that participants needed to complete the L-span task at the five different presentation levels, while they only completed the R-span task once. The L-span approach was adopted to prevent the task from being too time-consuming, frustrating or unpleasant for participants (Conway et al., 2005). L-span served as our criterion measure of auditory working memory span.
Procedure
Participants were provided with informed consent and then they completed a demographic questionnaire obtaining information such as gender, age, perceived hearing ability, and current medication usage. Next, participants completed the pure-tone audiometric screening, the speech reception threshold assessments and the Trail Making Test – Part B. Participants then completed the R-span and the L-span tasks in counterbalanced order. Participants were provided with practice on both span tasks prior to beginning the scored trials.
Results and Discussion
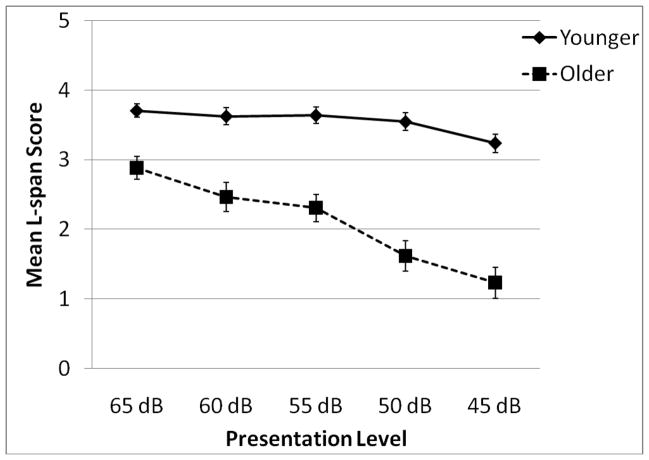
In order to investigate whether the presentation level had differential effects on L-span performance for the younger and older groups, we conducted a 2 (age group) X 5 (presentation level) split-plot multivariate analysis of variance on mean L-span scores. Results revealed a significant main effect of age group [F (1, 98) = 72.99, p < 0.001, partial-eegr;2 = .424] and significant main effect of presentation level [F (4, 95) = 15.69, p < 0.001, partial-η2 = .395]. These effects were subsumed under a significant age by presentation level interaction [F (4, 95) = 6.31, p < 0.001, partial-η2 = .209]. Means and standard errors for this effect are shown in Figure 1. To investigate the nature of this interaction a planned linear trend analyses on presentation level was conducted for each age group. While both groups showed a significant linear decrease in performance as presentation level decreased, the effect sizes show that the decreasing presentation level had a much larger impact on older participants’ performance [F(1, 25) = 48.06, p < .001, partial-η2 = .685] than it did on younger participants’ performance [F(1, 73) = 9.39, p < .01, partial-η2 = .113]. These results show that decreasing presentation level had a negative effect on auditory working memory performance in both younger and older adults, but it had greater detrimental impact on older adults.
Figure 1.
Mean listening span scores as a function of age group and presentation level (error bars represent standard error).
Next, we investigated the role that visual working memory capacity (R-span), processing speed (TMT-B task time), and speech reception threshold (SRT) played in this difference between younger and older participants’ auditory working memory performance at different presentation levels. We began by comparing older and younger participants’ performance on these tasks via a series of independent samples t-tests. Results revealed large significant differences between the groups on TMT-B time (younger adults M = 61.34 s, SD = 20.70; older adults M = 86.99 s, SD = 27.79, t(98) = −4.95, p < .001, Cohen’s d = −1.13) and SRT (younger adults M = 32.47 dBA, SD = 3.63; older adults M = 36.46 dBA, SD = 5.21, t(98) = −4.27, p < .001, Cohen’s d = −0.98), with younger participants performing better on each of these measures. However, younger participants’ advantage on the R-span task was much smaller and was not significantly different from the older participants’ performance (younger adults M = 0.72, SD = 0.14; older adults M = 0.66, SD = 0.13, t(98) = 1.90, p = .06, Cohen’s d = 0.43).
Next we conducted a series of correlational analyses to investigate the relationship between TMT-B, SRT, R-span, and L-span performance at the different presentation levels for the older and younger participants. For the purposes of these analyses, all measures were recoded so that positive correlations indicated that better performance on a covariate task was related to better performance of the L-span task. We conducted a multivariate analysis of covariance (MANCOVA) to investigate whether the unique relationships between the covariates and L-span performance varied as a function of age group and presentation level. In the MANCOVA analysis, we entered the main effects and interaction of the presentation level and age group variables, the three covariates, and all two and three-way interactions between the covariates and the presentation level and age group variables. In this analysis, main effects of the covariates represent their predictive value across age groups and presentation levels, while interactions indicate changes in the relationship of the covariates to L-span scores as a function of presentation level and age group. The standardized regression coefficients that correspond to this analysis are presented in Table 1.
Table 1.
Standardized regression coefficients (β) and two-tailed p-values from multiple regressions using processing speed (TMT-B), visual working memory span (R-span) and speech reception threshold (SRT) to predict auditory working memory span (L-span) as function of age group and presentation level.
| Presentation Level | TMT-B | R-span | SRT | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Younger adults | Older adults | Younger adults | Older adults | Younger adults | Older adults | |||||||
| β | p | β | p | β | p | β | p | β | p | β | p | |
| 65 dBA | 0.056 | .632 | 0.110 | .634 | 0.396 | .001 | 0.303 | .096 | −0.118 | .290 | 0.509 | .006 |
| 60 dBA | 0.177 | .150 | 0.007 | .905 | 0.293 | .077 | 0.134 | .363 | −0.070 | .549 | 0.736 | .000 |
| 55 dBA | 0.241 | .045 | −0.200 | .196 | 0.207 | .079 | 0.111 | .443 | 0.060 | .601 | 0.745 | .000 |
| 50 dBA | 0.225 | .057 | −0.042 | .967 | 0.154 | .182 | 0.153 | .334 | 0.219 | .054 | 0.692 | .000 |
| 45 dBA | 0.061 | .594 | −0.271 | .082 | 0.082 | .464 | −0.031 | .840 | 0.434 | .000 | 0.742 | .000 |
The age group X TBT-B interaction was the only effect of processing speed that approached traditional significance levels, indicating that across presentation levels TMT-B was more predictive of L-span performance for younger adults, F(1, 92) = 3.80, p = .054, partial-η2 = .04. For R-span, the results revealed a significant main effect indicating that across presentation levels and age groups R-span predicted auditory working span performance, F(1, 92) = 5.99, p = .016, partial-η2 = .06. However, no two or three-way interactions of R-span and the age group or presentation level variables were detected. There was a significant main effect of SRT [F(1, 92) = 49.53, p < .001, partial-η2 = .35], a significant age X SRT [F(1, 92) = 21.45, p < .001, partial-η2 = .19], and a significant presentation level X SRT [F(4, 89) = 6.44, p < .001, partial-η2 = .23]. These effects were subsumed under a significant age X presentation level X SRT, F(4, 89) = 3.03, p = .022, partial-η2 = .12. As shown in Table 1, for younger adults the relationship between SRT and auditory working memory performance was not significant at the loudest presentation level and steadily increased as presentation level lowered, Flinear(1, 70) = 24.44, p < .001, partial-η2 = .26. In older adults, SRT and L-span performance were strongly related at the loudest presentation level. There relationship increased at the at the next lowest presentation level and reached an asymptote at the lower levels, Fquadratic(1, 22) = 24.44, p = .023, partial-η2 = .21.
In summary, decreasing the presentation level of the L-span task materials had a larger detrimental effect on older adults’ auditory working memory span performance than it did for younger adults. Older adults showed large deficits in processing speed and speech reception threshold when compared to younger adults. However the difference between younger and older adults on the visual working memory span task was smaller and not statistically significant. Finally, correlational analyses revealed that the relationship of speech reception threshold to auditory working memory performance varied as a function of age and presentation level. For older adults, speech threshold was a strong and consistent predictor of auditory working memory span performance across presentation levels. For younger participants, speech reception threshold was not related to auditory working memory span performance at the highest presentation levels but emerged as a significant predictor as the presentation level decreased.
General Discussion
The results support a growing body of literature indicating that declining acuity plays an important explanatory role in age-related declines in cognitive abilities (Baldwin & Struckman-Johnson, 2002; Lunner & Sundewall-Thorén, 2007; McCoy et al., 2005; Pichora-Fuller et al., 1995; Rabbitt, 1968, 1990; Schneider et al., 2005; Schneider, Daneman, & Pichora-Fuller, 2002; Scialfa, 2002). The findings also underscore the importance of considering the interaction between sensory and cognitive processes in both audiometric and cognitive testing (Arlinger et al., 2009; Baldwin, 2002; Lunner et al., 2009; Pichora-Fuller, 2007; Scialfa, 2002). While the present results support previous investigations indicating a strong association between sensory acuity and cognitive test performance (Lindenberger & Baltes, 1994; Valentijn et al., 2005), they support a modality specific rather than common cause association (Valentijn et al., 2005; van Boxtel, ten Tusscher, Metsemakers, Willems, & Jolles, 2001; van Boxtel et al., 2000).
There are limitations of the current study that warrant further research. First, the relatively small sample size in the older group limited the power of the current analyses to detect moderate to small relationships among our variables. While this reduced power may explain why the moderate correlation of R-span and L-span at the highest presentation level or the difference between age groups on R-span did not reach traditional levels of statistical significance, it does not undermine the overall pattern of results as described here nor reduce the significance of our primary finding regarding the difference between the relationship of SRT and L-span performance in younger and older adults. Further, examination of scatter plots between SRT and auditory WM task performance for old and young adults confirmed that the results were not due to outliers. If anything, the small sample points out the magnitude of these effects and lends credence to the claim that sensory acuity may play a major role in age-related decline in cognitive task performance. A second issue comes from our findings that processing speed was not a strong predictor of age related differences in L-span performance. Part of this issue may be due to our use of the TMT-B task as our measure of processing speed. Although TMT-B correlates moderately well with other perceptual speed indices and has been used in previous research for this purpose (Burton et al., 2006; Salthouse, 1994), the manner in which it is administered does not allow for the computations of multiple indices necessary to access reliability. This leaves open the possibility that the small effects of processing speed found in the current study were due to low measurement reliability or problems with the construct validity of the TMT-B measure as an index of processing speed.
Despite these limitations, the results of the current investigation support a growing body of literature indicating that sensory acuity plays an increasingly strong role in cognitive performance as people age (McCoy et al., 2005; Pichora-Fuller, 2008; Schneider et al., 2002) and that poor quality stimulus material impacts speech understanding even in young adults when attentional demands are high, such as while trying to simultaneously drive (Baldwin & Struckman-Johnson, 2002) or hold information in memory.
Hearing impairment is a pervasive and global health concern that is more prevalent in, but not limited to older adults (Engdahl, Tambs, Borchgrevink, & Hoffman, 2005; Rabinowitz, Slade, Galusha, Dixon-Ernst, & Cullen, 2006). The present results indicate that performance of a task that place demands on working memory capacity, a fundamental component of many higher order processes, is negatively impacted by hearing impairment. At the same time, individuals with smaller working memory capacities are less able to compensate for either poor listening conditions or reduced or hearing abilities (Lunner, 2003). It may be some time before the relative contribution of each of these factors can be isolated; but, it is clear that both peripheral and central issues impact the speech understanding of older adults.
The results have some implications for everyday listening situations. Hearing impaired listeners could be expected to remember fewer details in a conversation and would likely have greater difficulty processing complex or lengthy discourse than their normal hearing peers. Unfortunately, this sensory challenge – though likely correctable – could be mistaken for cognitive impairment.
Acknowledgments
We wish to acknowledge financial support from the National Institutes of Health, National Institute of Aging, for grant # R03 AG023881-01 awarded to the first author.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/PAG
Contributor Information
Carryl L. Baldwin, George Mason University, Fairfax, VA
Ivan K. Ash, Old Dominion University, Norfolk, VA
References
- Addis DR, Wong AT, Schacter DL. Age-related changes in the episodic simulation of future events. Psychological Science. 2008;19(1):33–41. doi: 10.1111/j.1467-9280.2008.02043.x. [DOI] [PubMed] [Google Scholar]
- Arlinger S, Lunner T, Lyxell B, Pichora-Fuller MK. Background and basic processes: The emergence of cognitive hearing science. Scandinavian Journal of Psychology. 2009;50(5):371–384. doi: 10.1111/j.1467-9450.2009.00753.x. [DOI] [PubMed] [Google Scholar]
- ASHA. Guidelines for determining the threshold level for speech. (American Speech and Hearing Association) ASHA. 1979;21:353–356. [Google Scholar]
- Baldwin CL. Designing in-vehicle technologies for older drivers: Application of sensory-cognitive interaction theory. Theoretical Issues in Ergonomics Science. 2002;3(4):307–329. [Google Scholar]
- Baldwin CL. Cognitive Implications of Facilitating Echoic Persistence. Memory & Cognition. 2007;35(4):774–780. doi: 10.3758/bf03193314. [DOI] [PubMed] [Google Scholar]
- Baldwin CL, Struckman-Johnson D. Impact of speech presentation level on cognitive task performance: Implications for auditory display design. Ergonomics. 2002;45(1):61–74. doi: 10.1080/00140130110115336. [DOI] [PubMed] [Google Scholar]
- Bopp KL, Verhaeghen P. Working memory and aging: Separating the effects of content and context. Psychology and Aging. 2009;24(4):968–980. doi: 10.1037/a0017731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botwinick J. Aging and behavior. 3. New York: Springer; 1984. [Google Scholar]
- Burton CL, Strauss E, Hultsch DF, Hunter MA. Cognitive functioning and everyday problem solving in older adults. Clinical Neuropsychologist. 2006;20(3):432–452. doi: 10.1080/13854040590967063. [DOI] [PubMed] [Google Scholar]
- Cerella J. Information processing rates in the elderly. Psychological Bulletin. 1985;98(1):67–83. [PubMed] [Google Scholar]
- Conway ARA, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW. Working memory span tasks: A methodological review and user’s guide. Psychonomic Bulletin & Review. 2005;12(5):769–786. doi: 10.3758/bf03196772. [DOI] [PubMed] [Google Scholar]
- Corso JF. Aging and auditory thresholds in men and women. Archives of Environmental Health. 1963;6:350–356. doi: 10.1080/00039896.1963.10663405. [DOI] [PubMed] [Google Scholar]
- Daneman M, Carpenter PA. Individual differences in working memory and reading. Journal of Verbal Learning & Verbal Behavior. 1980;19(4):450–466. [Google Scholar]
- Daneman M, Carpenter PA. Individual differences in integrating information between and within sentences. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1983;9(4):561–584. [Google Scholar]
- de Frias CM, Dixon RA, Fisher N, Camicioli R. Intraindividual variability in neurocognitive speed: A comparison of Parkinson’s disease and normal older adults. Neuropsychologia. 2007;45(11):2499–2507. doi: 10.1016/j.neuropsychologia.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Engdahl B, Tambs K, Borchgrevink HM, Hoffman HJ. Screened and unscreened hearing threshold levels for the adult population: Results from the Nord-Trondelag Hearing Loss Study. International Journal of Audiology. 2005;44(4):213–230. doi: 10.1080/14992020500057731. [DOI] [PubMed] [Google Scholar]
- Engle RW, Kane MJ, Tuholski SW. Individual differences in working memory capacity and what they tell us about controlled attention, general fluid intelligence, and functions of the prefrontal cortex. In: Miyake A, Shah P, editors. Models of working memory: Mechanisms of active maintenance and executive control. New York, NY: Cambridge University Press; 1999. pp. 102–134. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-Mental-State’. A practical method for grading the cognitive state of patients for the clinicians. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fozard JL, Gordon-Salant S. Changes in vision and hearing with aging. In: Birren JE, editor. Handbook of the psychology of aging. San Diego, CA: Academic Press; 2001. pp. 241–266. [Google Scholar]
- Gates GA, Cooper JC, Kannel WB, Miller NJ. Hearing in the elderly: The Framingham Cohort, 1983–1985. Part 1. Basic audiometric test results. Ear and Hearing. 1990;11(4):247–256. [PubMed] [Google Scholar]
- Glass JM, Schumacher EH, Lauber EJ, Zurbriggen EL, Gmeindl L, Kieras DE, et al. Aging and the psychological refractory period: Task-coordination strategies in young and old adults. Psychology & Aging. 2000;15(4):571–595. doi: 10.1037//0882-7974.15.4.571. [DOI] [PubMed] [Google Scholar]
- Helzner EP, Cauley JA, Pratt SR, Wisniewski SR, Zmuda JM, Talbott EO, et al. Race and Sex Differences in Age-Related Hearing Loss: The Health, Aging and Body Composition Study. Journal of the American Geriatrics Society. 2005;53(12):2119–2127. doi: 10.1111/j.1532-5415.2005.00525.x. [DOI] [PubMed] [Google Scholar]
- Humes LE. The contributions of audibility and cognitive factors to the benefit provided by amplified speech to older adults. Journal of the American Academy of Audiology. 2007;18(7):590–603. doi: 10.3766/jaaa.18.7.6. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Bleckley MK, Conway ARA, Engle RW. A controlled-attention view of working-memory capacity. Journal of Experimental Psychology: General. 2001;130(2):169–183. doi: 10.1037//0096-3445.130.2.169. [DOI] [PubMed] [Google Scholar]
- Kliegel M, Jager T. Delayed-Execute Prospective Memory Performance: The Effects of Age and Working Memory. Developmental Neuropsychology. 2006;30(3):819–843. doi: 10.1207/s15326942dn3003_4. [DOI] [PubMed] [Google Scholar]
- Kliegel M, Jager T, Phillips LH. Adult age differences in event-based prospective memory: A meta-analysis on the role of focal versus nonfocal cues. Psychology and Aging. 2008;23(1):203–208. doi: 10.1037/0882-7974.23.1.203. [DOI] [PubMed] [Google Scholar]
- Kray J, Eber J, Lindenberger U. Age differences in executive functioning across the lifespan: The role of verbalization in task preparation. Acta Psychologica. 2004;115(2–3):143–165. doi: 10.1016/j.actpsy.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Li L, Daneman M, Qi JG, Schneider BA. Does the Information Content of an Irrelevant Source Differentially Affect Spoken Word Recognition in Younger and Older Adults? Journal of Experimental Psychology: Human Perception & Performance. 2004;30(6):1077–1091. doi: 10.1037/0096-1523.30.6.1077. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: A strong connection. Psychology & Aging. 1994;9(3):339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- Lunner T. Cognitive function in relation to hearing aid use. International Journal of Audiology. 2003;42(Suppl1):S49–S58. doi: 10.3109/14992020309074624. [DOI] [PubMed] [Google Scholar]
- Lunner T, Rudner M, Ronnberg J. Background and basic processes: Cognition and hearing aids. Scandinavian Journal of Psychology. 2009;50(5):395–403. doi: 10.1111/j.1467-9450.2009.00742.x. [DOI] [PubMed] [Google Scholar]
- Lunner T, Sundewall-Thorén E. Interactions between Cognition, Compression, and Listening Conditions: Effects on Speech-in-Noise Performance in a Two-Channel Hearing Aid. Journal of the American Academy of Audiology. 2007;18(7):604. doi: 10.3766/jaaa.18.7.7. [DOI] [PubMed] [Google Scholar]
- McCoy SL, Tun PA, Cox L, Colangelo M, Stewart RA, Wingfield A. Hearing loss and perceptual effort: Downstream effects on older adults’ memory for speech. Quarterly Journal of Experimental Psychology: Human Experimental Psychology. 2005;58A(1):22–33. doi: 10.1080/02724980443000151. [DOI] [PubMed] [Google Scholar]
- Newby HA. Audiology. Englewood Cliffs, NJ: Prentice-Hall; 1972. [Google Scholar]
- Old SR, Naveh-Benjamin M. Differential effects of age on item and associative measures of memory: A meta-analysis. Psychology and Aging. 2008;23(1):104–118. doi: 10.1037/0882-7974.23.1.104. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychology and Aging. 2002;17(2):299–320. [PubMed] [Google Scholar]
- Pichora-Fuller M. Rehabilitative Audiology: Using the Brain to Reconnect Listeners with Impaired Ears to Their Acoustic Ecologies. Journal of the American Academy of Audiology. 2007;18(7):536. [Google Scholar]
- Pichora-Fuller MK. Use of supportive context by younger and older adult listeners: balancing bottom-up and top-down information processing. International Journal of Audiology. 2008;47(S2):S72–82. doi: 10.1080/14992020802307404. [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Schneider BA, Daneman M. How young and old adults listen to and remember speech in noise. Journal of the Acoustical Society of America. 1995;97(1):593–608. doi: 10.1121/1.412282. [DOI] [PubMed] [Google Scholar]
- Rabbitt PM. Channel-capacity, intelligibility and immediate memory. The Quarterly Journal of Experimental Psychology. 1968;20(3):241–248. doi: 10.1080/14640746808400158. [DOI] [PubMed] [Google Scholar]
- Rabbitt PM. Mild hearing loss can cause apparent memory failures which increase with age and reduce with IQ. Acta Oto-Laryngologica Supplementum. 1990;476:167–175. doi: 10.3109/00016489109127274. [DOI] [PubMed] [Google Scholar]
- Rabinowitz PM, Slade MD, Galusha D, Dixon-Ernst C, Cullen MR. Trends in the prevalence of hearing loss among young adults entering an industrial workforce 1985 to 2004. Ear and Hearing. 2006;27(4):369–375. doi: 10.1097/01.aud.0000224125.12338.9a. [DOI] [PubMed] [Google Scholar]
- Reitan RM. The relation of the Trail Making Test to organic brain damage. Journal of Consulting Psychology. 1955;19(5):393–394. doi: 10.1037/h0044509. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Salthouse TA. Working-memory mediation of adult age differences in integrative reasoning. Memory & Cognition. 1992;20(4):413–423. doi: 10.3758/bf03210925. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The nature of the influence of speed on adult age differences in cognition. Developmental Psychology. 1994;30(2):240–259. doi: 10.1037//0278-7393.20.6.1486. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Memory aging from 18 to 80. Alzheimer Disease & Associated Disorders. 2003;17(3):162–167. doi: 10.1097/00002093-200307000-00008. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Toth J, Daniels K, Parks C, Pak R, Wolbrette M, et al. Effects of aging on efficiency of task switching in a variant of the Trail Making Test. Neuropsychology. 2000;14(1):102–111. [PubMed] [Google Scholar]
- Sarampalis A, Kalluri S, Edwards B, Hafter E. Objective Measures of Listening Effort: Effects of Background Noise and Noise Reduction. Journal of Speech, Language & Hearing Research. 2009;52(5):1230–1240. doi: 10.1044/1092-4388(2009/08-0111). [DOI] [PubMed] [Google Scholar]
- Schieber F, Baldwin CL. Vision, audition, and aging research. In: Blanchard-Fields F, Hess TM, editors. Perspectives on Cognitive Change in Adulthood and Aging. New York: McGraw-Hill; 1996. [Google Scholar]
- Schneider BA, Daneman M, Murphy DR. Speech Comprehension Difficulties in Older Adults: Cognitive Slowing or Age-Related Changes in Hearing? Psychology and Aging. 2005;20(2):261–271. doi: 10.1037/0882-7974.20.2.261. [DOI] [PubMed] [Google Scholar]
- Schneider BA, Daneman M, Pichora-Fuller MK. Listening in aging adults: From discourse comprehension to psychoacoustics. Canadian Journal of Experimental Psychology. 2002;56(3):139–152. doi: 10.1037/h0087392. [DOI] [PubMed] [Google Scholar]
- Schneider BA, Pichora-Fuller MK. Implications of perceptual deterioration for cognitive aging research. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 2. Mahwah, N.J: Lawrence Erlbaum; 2000. [Google Scholar]
- Scialfa CT. The role of sensory factors in cognitive aging research. Canadian Journal of Experimental Psychology/Revue canadienne de psychologie expérimentale. 2002;56(3):153–163. doi: 10.1037/h0087393. [DOI] [PubMed] [Google Scholar]
- Tschopp K, Beckenbauer T, Harris FP. Objective measures of sentence level with respect to loudness. Audiology. 1991;30(2):113–122. doi: 10.3109/00206099109072876. [DOI] [PubMed] [Google Scholar]
- Turner ML, Engle RW. Is working memory capacity task dependent? Journal of Memory and Language. 1989;28(2):127–154. [Google Scholar]
- Valentijn SAM, van Boxtel MPJ, van Hooren SAH, Bosma H, Beckers HJM, Ponds RWHM, et al. Change in Sensory Functioning Predicts Change in Cognitive Functioning? Results from a 6-Year Follow-Up in the Maastricht Aging Study. Journal of the American Geriatrics Society. 2005;53(3):374–380. doi: 10.1111/j.1532-5415.2005.53152.x. [DOI] [PubMed] [Google Scholar]
- van Boxtel MPJ, ten Tusscher MPM, Metsemakers JFM, Willems B, Jolles J. Visual determinants of reduced performance on the Stroop Color-Word Test in normal aging individuals. Journal of Clinical and Experimental Neuropsychology. 2001;23(5):620–627. doi: 10.1076/jcen.23.5.620.1245. [DOI] [PubMed] [Google Scholar]
- van Boxtel MPJ, van Beijsterveldt CEM, Houx PJ, Anteunis LJC, Metsemakers JFM, Jolles J. Mild hearing impairment can reduce verbal memory performance in a healthy adult population. Journal of Clinical and Experimental Neuropsychology. 2000;22(1):147–154. doi: 10.1076/1380-3395(200002)22:1;1-8;FT147. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Salthouse TA. Meta-analyses of age-cognition relations in adulthood: Estimates of linear and nonlinear age effects and structural models. Psychological Bulletin. 1997;122(3):231–249. doi: 10.1037/0033-2909.122.3.231. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Steitz DW, Sliwinski MJ, Cerella J. Aging and dual-task performance: A meta-analysis. Psychology & Aging. 2003;18(3):443–460. doi: 10.1037/0882-7974.18.3.443. [DOI] [PubMed] [Google Scholar]
- Villaume WA, Brown MH, Darling R. Presbycusis, communication and older adults. In: Hummert ML, Wiemann JM, Nussbaum JF, editors. Interpersonal Communication in Older Adulthood. Interdisciplinary Theory and Research. London: Sage; 1994. [Google Scholar]
- Wingfield A, Tun PA, McCoy SL. Hearing Loss in Older Adulthood. What it is and how it interacts with cognitive performance. Current Directions in Psychological Science. 2005;14(3):144–148. [Google Scholar]