Abstract
Social status and resource availability can strongly influence individual behavioral responses to conspecifics. In European starlings, males that acquire nest sites sing in response to females and dominate other males. Males without nest sites sing, but not to females, and they do not interact agonistically with other males. Little is known about the neural regulation of status- or resource-appropriate behavioral responses to conspecifics. Opioid neuropeptides are implicated in birdsong and agonistic behavior, suggesting that opioids may underlie differences in the production of these behaviors in males with and without nest sites. Here, we examined densities of immunolabeled mu-opioid receptors in groups of male starlings. Males that defended nest boxes dominated other males and sang at higher rates when presented with a female than males without nest boxes, independent of testosterone concentrations. Multiple regression analyses showed nest box ownership (not agonistic behavior or singing) predicted the optical density of receptor labeling in the medial bed nucleus of stria terminalis, paraventricular nucleus, ventral tegmental area and the medial preoptic nucleus. Compared to males without nest boxes, males with nest boxes had lower densities of immunolabeled mu-opioid receptors in these regions. Singing additionally predicted the area covered by labeling in the ventral tegmental area. The results suggest that elevated opioid activity in these regions suppresses courtship and agonistic behavioral responses to conspecifics in males without nest boxes. The findings are consistent with a dynamic role for opioid receptors in adjusting social behavior so that it is appropriate given the resources available to an individual.
Keywords: birdsong, motivation, vocal communication, socially appropriate behavior, dominance, territoriality
1. Introduction
Across vertebrates both social status and the resources available to an individual can strongly influence the way in which an individual responds to conspecifics. For example, in male European starlings (Sturnus vulgaris), the acquisition and defense of a nest site (or nest box in aviary studies) is a crucial first step for initiating mating behavior [1]. Male starlings with nest boxes sing high rates of song in response to females (Riters et al., 2000). In contrast, male starlings that do not successfully obtain nest boxes often sing, but not in response to a female [2]. Males with nest boxes additionally displace other males from food sources and perches more often than males without nest boxes, suggesting nest box owners socially dominate males without boxes [3]. Thus, the distinct behavioral responses to conspecifics observed in males with and without nest boxes are established early in the breeding season through competition over limited available nest sites. Little is known about how social status or resource possession alters the brain to ensure that an individual produces status- or resource-appropriate behavioral responses to conspecifics. In the present study we begin to explore a possible role for opioids in social behavior associated with nest box possession in flocks of male starlings.
Data implicate opioids in the regulation of birdsong and suggest that the effects of opioids on song differ depending upon whether song is directed towards a female (as in nest box owners) or undirected (as in males without nest boxes). In male starlings with nest boxes, opioids acting at the mu-opioid receptor inhibit courtship song produced in response to a female ([4, 5], but see Khurshid et al., 2009 for different effects in zebra finches). Furthermore, male starlings that sang at naturally high or low rates responded differently to opioid receptor pharmacological treatments. For example, treatment with the opioid receptor antagonist naloxone increased song production in low singers, but did not have a dramatic effect on high singers [6]. This suggests differences in mu-opioid receptor density or binding affinity may underlie individual differences in males singing at high and low rates in response to a female (i.e., in males with and without nest boxes respectively).
Studies using immunolabeling for immediate early genes (IEGs) identify differences in neuronal activity in males with compared to males without nest boxes. Specifically, males with nest boxes had higher numbers of IEG labeled cells in regions implicated in both agonistic behavior and birdsong, including the medial preoptic nucleus (POM; see Table 1 for abbreviations), medial bed nucleus of the stria terminalis (BSTm), ventral tegmental area (VTA), and mesencephalic central gray (CG) [7–11]. Each of these regions contains opioids or opioid receptors [5, 12–15]. In male starlings, immunolabeling for the opioid met-enkephalin in the POM and VTA correlated with male song production, however for POM this correlation existed only for song that was not produced in response to a female (with a similar trend observed for VTA) [5]. These results link opioids in the POM and VTA specifically to the type of song produced by males without nest boxes. The results of opioid-receptor pharmacological manipulations in male zebra finches also support the idea that opioids more strongly influence undirected song compared to female-directed song [15].
Table 1.
List of Abbreviations
| Abbreviation | Full Name |
|---|---|
| BSTm | Bed nucleus of the stria terminalis, medial part |
| CG | Midbrain central gray |
| LS | Lateral septum |
| POM | Medial preoptic nucleus |
| PVN | Paraventricular nucleus |
| T | Testosterone |
| VTA | Ventral tegmental area |
Altogether, the results of pharmacology studies and met-enkephalin immunolabeling suggest that the effects of opioids in part acting within POM and VTA may differ in males singing at high rates in response to a female (i.e., males with nest boxes) compared to those singing low rates of undirected song (i.e., males without nest boxes). To test this, we examined individual differences in densities of immunolabeled mu-opioid receptors in flocks of male starlings in aviaries with nest boxes. If the inhibitory role of opioids in female-directed song in starlings is mediated by opioids in POM and VTA, then we predict that mu-opioid receptor densities will be lower in these and possibly other regions in males that successfully acquire nest boxes compared to those that fail to obtain a nest box.
2. Material and Methods
2.1 Animals
In January of 2008, 19 adult male and 10 adult female starlings were captured on a single farm west of Madison, Wisconsin using baited fly-in traps. After capture, birds were immediately housed indoors in stainless steel, single sex cages (91 cm×47 cm×47 cm) within the University of Wisconsin’s Department of Zoology indoor animal facilities. Food (Purina Mills Start and Grow Sunfresh Recipe, 61S3-IGH-G) and water were provided ad libitum. Each animal was assigned a numbered and colored leg band for identification. All procedures and protocols were in adherence with the Food and Drug Administration’s Guide and in accordance with the University of Wisconsin-Madison Research Animal Resource Committee (RARC). All procedures were approved by the University of Wisconsin Institutional Animal Care and Use Committee.
Prior to study initiation, a hackle feather was removed from the breast of each bird and the length of iridescence was measured using calibrated calipers. Previous research [16] shows that males with hackle feathers ranging from 11–15mm are older than one year of age. Based on hackle feather length, all birds in the present study were estimated to be over one year of age at the start of the experiment.
2.2 Light cycle and hormone manipulations
Prior to study initiation, birds were housed indoors and placed on artificial photoperiods of 18L: 6D for six weeks, followed by a photoperiod of 6L: 18D for an additional six weeks. These photoperiod manipulations induce photosensitivity, a condition in which when given T implants male starlings with nest boxes display high rates of courtship song and behaviors in response to a female [17, 18]. Each male test subject received two subcutaneous implants of testosterone (T) (as detailed below). Each stimulus female also received two subcutaneous implants of 17β-estradiol, to maximize female sexual interest in males in the laboratory.
Hormones were implanted seventeen days prior to the first day of behavioral testing. All birds were lightly anesthetized using isoflurane gas anesthesia and a small pocket-like incision was made in the skin over the breast muscle. The incision site was sutured closed (Ethilon nylon suture, 13mm, 3.8c, #698G). Males received two, 14-mm lengths of silastic tubing (i.d., 1.47-mm; o.d. 1.96-mm; Dow Corning, Midland, MI) packed for 10-mm with crystalline testosterone proprionate (Sigma-Aldrich, St. Louis, MO). Females received two, 17-mm lengths of silastic tubing packed for 13-mm with 17β-estradiol (Sigma-Aldrich). After recovering on a heated pad, male test subjects were placed in outdoor aviaries on natural day length and allowed to acclimate to study conditions. Female stimulus birds were returned to their home cages.
2.3 Housing and acclimation
Animals were randomly assigned to one of five outdoor aviaries (4 birds per aviary; 2.13m × 2.4m × 1.98m per aviary) and allowed to habituate prior to the beginning of behavioral testing. Each aviary contained four nest boxes and branches for perching. Food and water were provided ad libitum. Each aviary was visually, but not acoustically isolated from the others by the use of camouflage blinds. Each day during the week prior to the first day of behavioral observations, green nest materials (green grass clippings and leaves) and a novel female were introduced into each aviary to allow males to habituate to the study procedures as well as the presence of an observer. During this time the observer additionally, practiced observing each male starling. Starlings display high site fidelity and behavioral data can reliably be collected from multiple birds in a single aviary simultaneously [17, 19, 20].
2.4 Behavioral observations and study procedures
Over five consecutive days, each aviary was provided with nest material and one stimulus female (a novel female each day) was released into the aviary. Five minutes after the introduction of the female, each aviary was observed for 20min by a single observer concealed by dark green camouflage. The order of observations across aviaries was randomized each day, with observations performed between 8:00 and 13:00. Starling song is complex and consists of at least four distinct components including introductory whistles, complex phrases, click series, and high-frequency phrases [21]. During each observation period the total number of complete song bouts (i.e., bouts containing each of the 4 components) was recorded. A distinct bout was defined as an event separated from the next event by at least two seconds. Furthermore, nest box directed behaviors were recorded, including the number of times males entered and exited nest boxes, collected nesting material and looked in the nest box. Additionally, measures of agonistic behavior (including chasing and displacements from perches or food dishes) as well as bouts of wing waving, feeding, and drinking were also recorded.
2.5 Tissue collection and processing
Immediately after the last twenty-minute observation period, all males in a group were sacrificed via rapid decapitation. Each male was checked to confirm the presence of hormone implants. Brains were removed by dissection, fixed and agitated in a 5% acrolein solution overnight, rinsed and placed in a 30% sucrose cryoprotectant for 4 days, rapidly frozen with crushed dry ice, and stored at − 80 °C until sectioning. Using a cryostat, brains were cut in the coronal plane in three, 40µm series (each series contained every third section) and stored in anti-freeze cryoprotectant (PBS, polyvinylpyrrolidone, sucrose and ethylene glycol mixture) until processing. Storage in a cyroprotectant allows for the long term storage of free floating sections to reduce the loss of antigenicity [22]. Series one was used for mu-opioid receptor immunohistochemistry described here.
2.6 Blood sampling and serum analysis
As part of a separate study not reported here, a single sample of no more than 200uL of blood was collected via venipuncture of the ulnar vein of each male once during the five days of testing. A terminal trunk blood sample was taken immediately after sacrifice for use in the present study.
To insure that T concentrations were elevated by the implants, T plasma metabolites in the terminal blood sample were measured with a commercial grade competitive assay immunoassay (EIA; Cayman Chemical, Ann Arbor, MI, USA, Catalog No. 582701). Samples were run in duplicate, using the manufacture’s protocol at a dilution of 1:8 (determined in pilot studies as the optimal concentration) in buffer solution and visualized at 405nm with a BioTek 800 plate reader (#7331000, ELv800™, BioTek Instruments, Inc. Winooski, VT, USA). Sensitivities of the commercial EIA according to the manufacture’s specifications indicate a limit of detection: 80% B/B0: 6 pg/ml and sensitivity: 50% B/B0: 32 pg/ml. The assay is specific (cross reactivity to 5 α-DHT is 27.4%, and to 17- β -estradiol <.01 %). All samples were run within a single assay. The intraassay CV was 12.39%.
2.7 Antibody specificity tests
Brain tissue was processed using immunohistochemistry for the mu-opioid receptor. Antibody specificity was verified using multiple techniques. First, a preadsorption test (tissue was incubated in mu-opioid receptor antibody (Abcam, ab10275) with the mu-opioid receptor peptide (1:50; Abcam, ab46988)) revealed no labeling; additionally, in pilot studies that omitted the primary antibody no labeling was detected. Secondly, a Western blot analysis was run to confirm specificity. Snap frozen dissected brain blocks from the hypothalamus were homogenized after adding 300 µ L ice-cold lysis buffer consisting of 50 mM Tris-HCl, 1% Na-deoxycholate, 0.25% Nonidet P-40, 150 mM NaCl, 1 mM EDTA, protease inhibitor cocktail (P-8340, as directed; Sigma, St. Louis, Missouri, USA) and phosphatase inhibitor cocktail (P-2850, as directed, Sigma, St. Louis, Missouri, USA). In order to remove cellular debris and nuclei, the samples were centrifuged at 14000 r.p.m. for 10min at 4°C. The supernatant was collected and protein concentration determined by a BCA protein assay (Cat# 23225, Thermo Fisher Scientific, Rockford, IL, USA) . Fifteen micrograms of total protein from each animal were gel electrophoresed using a precast SDS-PAGE 4–20% Tris Glycine gel (Cat# 58645, Cambrex Bio Science Rockland, Inc., Rockland, Maine, USA) and transferred to a polyvinyl difluoride membrane (Immobilon-P; Cat# IPVH20200 Millipore, Bedford, Massachusetts, USA). Membranes were blocked for one hour in 0.1 M TBS containing 5% nonfat dry milk with constant agitation at room temperature. Membranes were then incubated overnight at 4°C with agitation in TBS containing 0.05% Tween-20 (TBS-T) and 2% nonfat dry milk with primary antibody (for mu-opioid receptor, Ab10275 [Abcam, 1:5000]) overnight at 4°C. The next day, the membranes were given three quick washes and then four five minute washes in TBS-T. Following washes, membranes were incubated in a goat anti-rabbit horseradish peroxidase-linked secondary antibody (Cat# 7074, Cell Signaling Technology, Inc., Beverly, Massachusetts, USA, 1:3000) and horseradish peroxidase conjugated anti-biotin antibody (Cat# 7075, Cell Signaling Technology, Inc., Beverly, Massachusetts, USA, 1:5000) for 30min at room temperature with agitation and then washed three times for 5min each with TBS-T and twice for 5min each with TBS. Immunoreactive bands were detected using a chemiluminisense kit (Ca# 7003, Cell Signaling Technology, Inc., Beverly, Massachusetts, USA) and exposed to X-ray film (Cat #178 8207, Eastman Kodak Co., Rochester, New York, USA).
Western immunoblot indicated a dark band at 48kDa, the molecular weight for the mu-opioid receptor protein [14, 23]. Two additional bands were observed at approximately 50 and 160 kDa. These bands were lighter and may represent splice variants [24, 25] or dimeric forms of the mu-opioid receptor due to glycosylation [26].
2.8 Immunohistochemistry
Immunohistochemistry on tissue from all males (n = 19) was run in a single batch and background labeling was observed to be similar across all sections. Sections were rinsed in phosphate buffered saline (PBS) for 30 min, incubated in 0.5% sodium borohydride solution for 15 min, rinsed in PBS for 20 min, incubated in 0.5% hydrogen peroxide solution for 10 min, rinsed in PBS for 20 min, incubated in 20% normal goat serum (NGS (made in PBS with 0.2% triton (PBS-T))) solution for 1 h, and then incubated in 2% NGS (made in PBS-T) primary solution overnight at room temperature (rabbit anti-mu-opioid receptor at 1:5000 (Abcam, ab10275)). Sections were then rinsed in PBS-T for 30 min and incubated in 2% NGS (made in PBS-T) biotinylated secondary solution for 90 min at room temperature (goat anti-rabbit at 1:1000 (Vector Laboratories, Burlingame, CA)). Sections were then rinsed in PBS-T for 30 min, incubated in AB solution (Vectastain Elite ABC, Vector Laboratories) for 1 h, rinsed in PBS-T for 30 min, and the avidin–biotin complex was visualized using 3,3′-Diaminobenzidine (DAB) tablets (Sigma Aldrich, St. Louis, MO, USA). Sections were float mounted onto gel-coated slides, dehydrated in a series of alcohols, and cover slipped.
2.9.1 Quantification
Using a Spot Camera (Diagnostic Instruments, Inc., Sterling Heights, MI, USA) connecting a Nikon microscope to a computer to acquire images of brain sections and using METAVUE (Fryer Company, Inc., Huntley, IL, USA) software, mean optical density and pixel area for mu-opioid receptor labeling were quantified bilaterally on three serial sections for each bird in regions implicated in reward, agonistic, and sexual behavior (POM, VTA, CG, LS, and BSTm). The locations of these nuclei were based on Heimovics and Riters (2007). Additionally, we took bilateral measures of labeling in the paraventricular nucleus (PVN) of the hypothalamus from three serial sections containing the anterior commissure. This region was included because it was rich in mu-opioid receptor labeling, and past work implicates PVN in sociosexual behaviors [27]. Labeling was not observed at high levels in areas in the nidopallium or arcopallium, thus measurements were not made in song control regions.
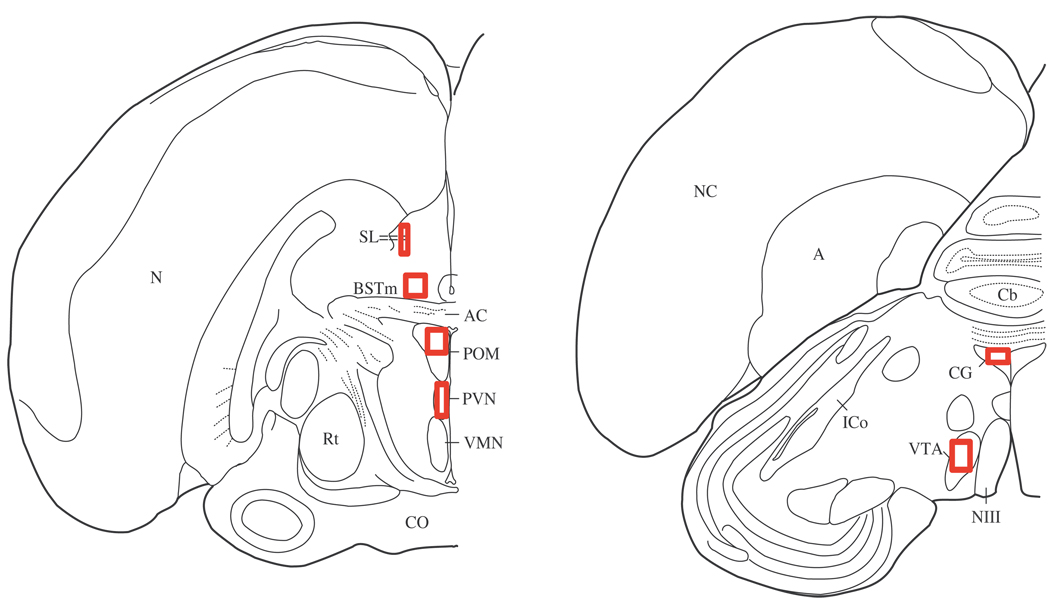
For measures of optical density and pixel area, a single computer generated threshold selected material that a blind observer agreed represented labeling. A separate threshold was generated for each brain region of interest. The METAVUE autoscale function was used to calculate the correct exposure of each image as a percentage of the total range of light, which further reduced the variation in background among individuals. Optical density is a measure of how much light is transmitted through the tissue, providing an index of labeling density. Pixel area (pixels highlighting fibers using the computer generated threshold) provides a measure of the approximate area covered by labeled receptors. Measures in POM, VTA, CG, PVN, LS, and BSTm were made within boxes or ovals (Figure 1; Table 2) centered within each region of interest, and measures were generated by the METAVUE software in each section in both hemispheres for each bird. In cases of tissue damage, labeling was quantified either on a fourth section or the individual was dropped from analysis for affected brain areas.
Figure 1.
Brain nuclei measurement locations. Boxes indicate approximate areas in which optical density and pixel area of mu-opioid receptor labeling was quantified. Abbreviations: A, arcopallium; BSTm, medial bed nucleus of the stria terminalis; Cb, cerebellum; AC, anterior commissure; CO, optic chiasm; CG, medial central gray; ICo, nucleus intercollicularis; NIII, third cranial nerve; N, nidopallium; NC, caudal nidopallium; POM, medial preoptic nucleus; PVN, paraventricular nucleus of the hypothalamus; Rt, nucleus rotundus; LS, lateral septum, VMN, ventromedial nucleus; VTA, ventral tegmental area.
Table 2.
Box measurements
| Nucleus | Shape | Size (mm) |
|---|---|---|
| BSTm | Rectangle | Area= 0.67 × 0.37 |
| CG | Oval | Diameter= 0.60 |
| POM | Rectangle | Area= 0.43 × 0.44 |
| PVN | Rectangle | Area= 0.22 × 0.42 |
| LS | Rectangle | Area= 0.36 × 0.54 |
| VTA | Rectangle | Area= 0.38 × 0.53 |
2.9.2 Statistical analysis
Data were analyzed using Statistica 6.0 software (Stat Soft Inc., Tulsa, OK). Males were defined as either nest box owners or non-owners. Specifically, if a male was observed entering and exiting the same nest box(es) multiple times (more than 5 times) on more than one day, he was designated as a nest box owner. Birds that did not display any aspect of song (intro whistles, fragments, or full song) during the behavioral observation period were dropped from analysis (n=3, non-owners).
A multivariate analysis of variance (MANOVA) was run with total song, nest box directed behaviors, total agonistic behavior, and the sum of feeding plus drinking behavior entered as repeated measures dependent variables and nest box ownership as a categorical between subjects independent variable (males with nest boxes, n=7; males without nest boxes, n=9). Separate MANOVAs were run with mu-opioid receptor optical density and pixel area in POM, VTA, CG, BSTm, LS, and PVN entered as repeated measures dependent variables and nest box ownership as a categorical between subjects independent variable. For the MANOVAs on mu-opioid receptors, a mean substitution (mean of all other animals in the group) was used to replace missing data. Specifically, data were substituted for: one male with no box for the POM, one male with a nest box for LS, and three males with and one without a box for BSTm. Because four replacements were required for BSTm (tissue folded or tore from the ventricle in this area) the MANOVA was run with and without this region included. The results were similar; therefore, the BSTm is included in the analysis. Finally, because mu-opioid receptors differed in males with and without nest boxes we ran multiple regression analyses to determine what best explained variance in mu-opioid receptor optical density and pixel area. Specifically, in separate multiple regression analyses a brain region was entered as the dependent variable and total number of songs, total number of agonistic interactions, and whether or not a male possessed a nest box were entered as predictor variables. Multiple regression analyses were run on the original data set for each region with no mean replacement. Influential statistical outliers were removed from analyses, resulting in removal of one data point from BSTm pixel area measures. For multiple regression analyses backward and forward analyses were performed. In all cases except for BSTm results were identical. For BSTm backward regression resulted in the same (though not identical) significant effects. Results of the backward analysis are provided because this model best explained the data based on the highest adjusted R2, lowest standard error, and the best residual plots.
3. Results
3.1 Behavior
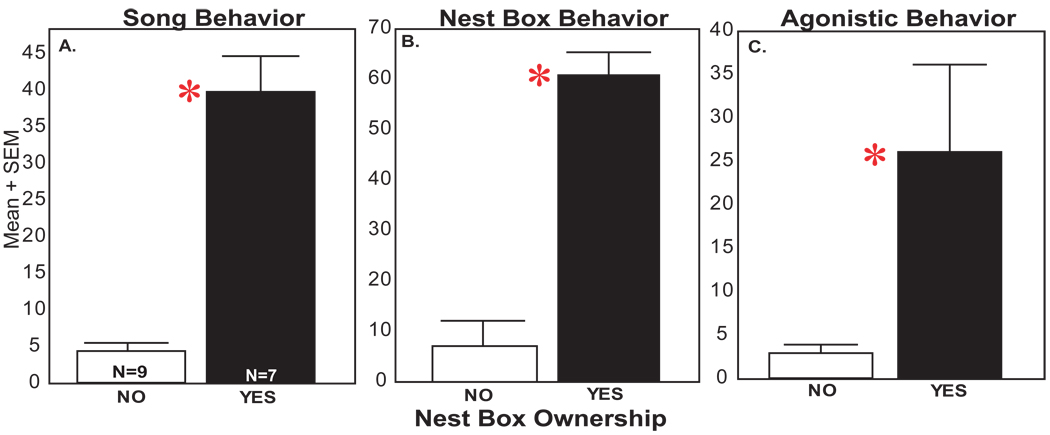
Sixteen males were used for behavioral analysis. Seven males obtained one or more nest boxes and nine failed to acquire a nest box. The results of a MANOVA revealed an overall effect for nest box ownership (F1, 14 = 45.59, p = 0.000009). Fischer’s post hoc analyses indicated that compared to males without nest boxes, nest box owners responded to the introduction of a female with significantly higher rates of total song (p = 0.0005; Figure 2A), displayed significantly more nest box directed behaviors (p = 0.000009; Figure 2B), and higher levels of agonistic behavior (the sum of all chases and displacements; p = 0.011; Figure 2C). Bouts of wing waving were only performed by nest box owners. Feeding and drinking behavior were not significantly different between males with or without nest boxes (p>0.05).
Figure 2.
Difference in behaviors between males with and without nest boxes. Mean number of behaviors in nest box owners, in black boxes, and non-owners in white for (A) Song, (B) Nest box specific behaviors (looking in the nest box, entering the nest box, collecting nest material), and (C) measures of agonistic behavior (chasing and displacements of conspecific males). Asterisk indicates significant differences between nest box owners and non-owners (p < 0.05).
3.2 Mu-opioid receptor labeling and nest box ownership
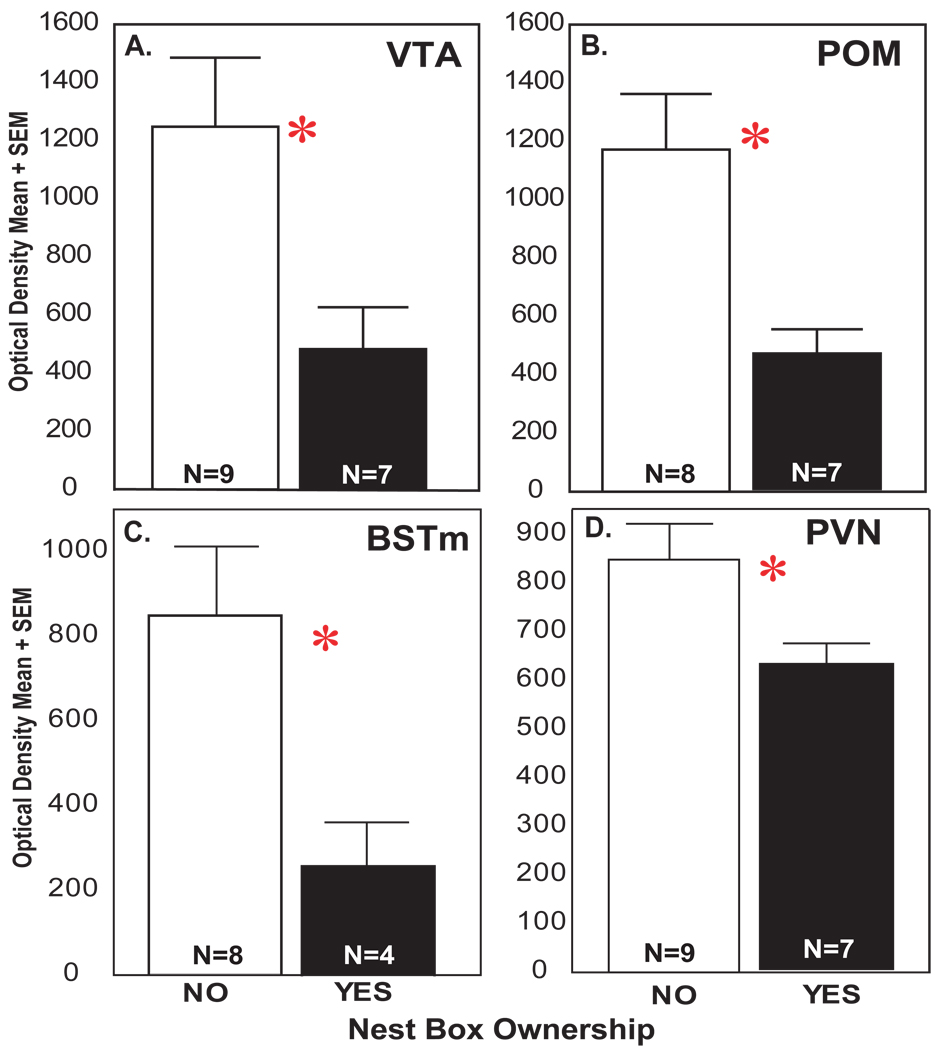
The results of a MANOVA revealed an overall effect for nest box ownership on both the optical density (F1,14 = 8.68, p = 0.01) and pixel area covered by labeled receptors (F1,14 = 8.26, p = 0.011). Specifically, mu-opioid receptor labeling area and density were significantly lower in males with compared to males without nest boxes (optical density shown in Figure 3; pixel area means in Table 3).
Figure 3.
Differences in mu-opioid receptor labeling in brain regions in males with and without nest boxes. Mean optical density of mu-opioid receptor labeling in (A) VTA, (B) POM, (C) BSTm, (D) PVN. Black bars represent males with nest boxes; white bars, males without nest boxes. Asterisk indicates a significant contribution of nest box ownership to mu-opioid receptor labeling density based on multiple regression analysis (p < 0.05).
Table 3.
Pixel Area Measurements (without mean data substitution)
| Brain Region | Nest box owners |
Owners Pixel Area Mean +/− SEM |
Nest box Non- owners |
Non-Owners Pixel Area Mean +/− SEM |
|---|---|---|---|---|
| VTA* | 7 | 9103.66 +/− 3206.408 | 9 | 28010.14 +/− 6502.517 |
| POM** | 7 | 4976.16 +/− 1366.120 | 8 | 11528.34 +/− 2227.134 |
| BSTm** | 4 | 4859.77 +/− 1415.464 | 8 | 16397.77 +/− 5684.518 |
| PVN | 7 | 18342.13 +/− 4061.620 | 9 | 19848.47 +/− 2138.746 |
| LS | 6 | 7429.17 +/− 1527.734 | 9 | 12099.39 +/− 2271.638 |
| CG | 7 | 17085.27 +/− 5289.357 | 9 | 26433.04 +/− 5474.350 |
indicates a significant contribution of singing behavior to mu-opioid receptor labeling density based on multiple regression analysis (p < 0.05).
indicates a significant contribution of nest box ownership to mu-opioid receptor labeling density based on multiple regression analysis (p < 0.05).
3.3 Mu-opioid receptor labeling and behavior
Results of multiple regression analyses revealed nest box ownership (and not total songs or agonistic interactions) to contribute significantly to variance in mu-opioid receptor optical density in BSTm, PVN, POM, and VTA (Figure 3 and 4; BSTm: adj R2 = 0.28, N = 12, nest box beta = 0.587, sd of beta = 0.26, t10 = 2.29, p = 0.045; PVN: adj R2 = 0.24, N = 16, nest box beta = 0.541, sd of beta = 0.22, t14 = 2.40, p = 0.031; POM: adj R2 = 0.40, N = 15, nest box beta = 0.668, sd of beta = 0.21, t13 = 3.24, p = 0.006; VTA: adj R2 = 0.27, N = 16, nest box beta = 0.566, sd of beta = 0.22, t14 = 2.56, p = 0.022). No variables contributed significantly to variance in LS or CG.
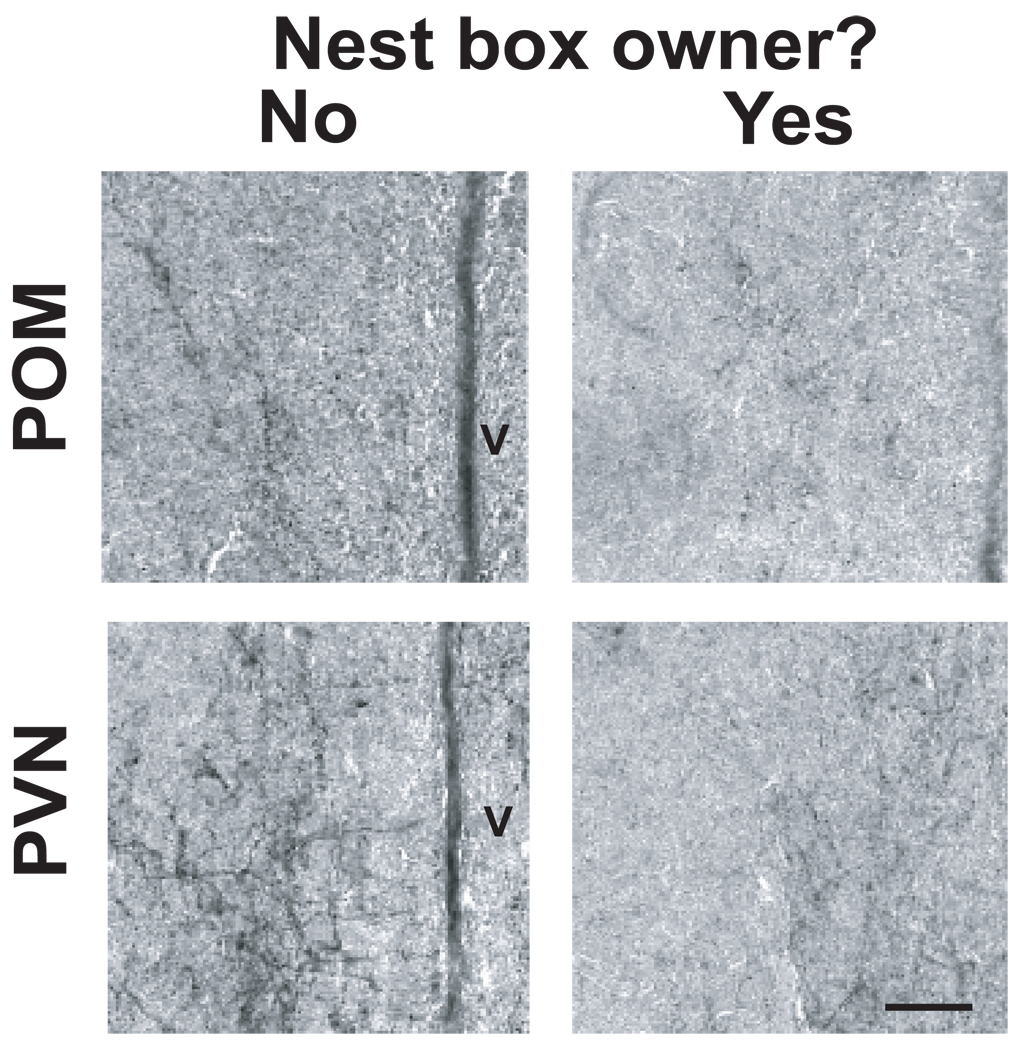
Figure 4.
Photomicrographs illustrating mu-opioid receptor labeling. Representative images include POM (top) and PVN (bottom) in males without (left) and with (right) nest boxes. Scale bar at the bottom right box is approximately .005mm.
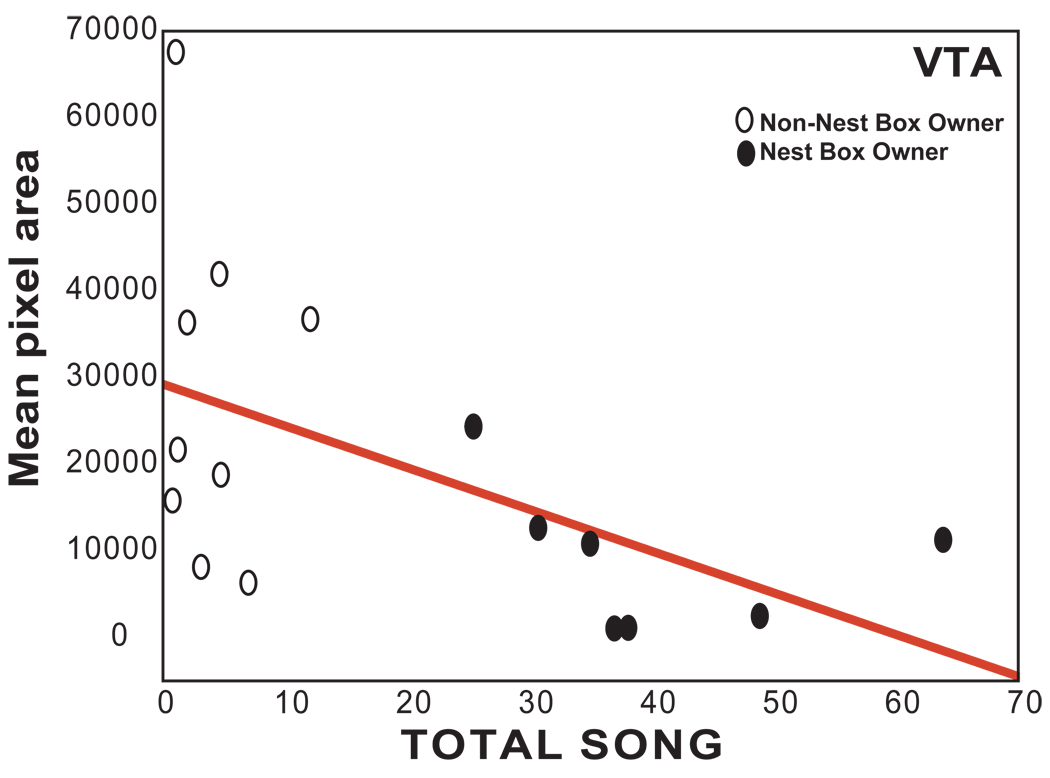
For measures of the pixel area covered by mu-opioid receptor labeling, results of multiple regression analyses revealed nest box ownership (and not total songs or agonistic interactions) to contribute significantly to variance in mu-opioid receptor pixel area in BSTm and POM (BSTm: adj R2 = 0.39, N = 11, nest box beta = 0.670, sd of beta = 0.25, t9 = 2.71, p = 0.024; POM: adj R2 = 0.26, N = 15, nest box beta = 0.557, sd of beta = 0.23, t13 = 2.71, p = 0.031; results of analyses of pixel area were similar to optical density and are thus not presented graphically but appear in Table 3). For VTA pixel area the total number of songs (rather than nest box ownership or agonistic behavior) contributed significantly (negatively) to variance in mu-opioid receptor pixel area (VTA: adj R2 = 0.24, N = 16, song beta = −0.540, sd of beta = 0.22, t14 = 2.40, p = 0.031; Table 3; Figure 5). No variables were found to contribute significantly to variance in pixel area in LS, CG or PVN.
Figure 5.
Scatterplot showing the relationship between pixel area measurements in VTA and song. Each point represents one individual, darkened circles represent males with nest boxes, open circles represent males without nest boxes. Presence of the regression line indicates a significant relationship (p<0.05).
3.4 Testosterone
An independent t test revealed no significant differences in T concentrations between males with or without nest boxes (with nest box: Mean= 0.08pg/ml, sd=0.04, without nest box: Mean=0.10 pg/ml, sd =0.07, p> 0.05).
4. Discussion
Consistent with past studies, males with and without nest boxes differed dramatically with respect to their behavioral responses to conspecifics [3, 4, 7, 28]. Males with nest boxes sang at high rates and accompanied song with wing waves (a courtship display); whereas, males without nest boxes sang at low rates and did not wing wave. Males with nest boxes also chased and displaced other males from perches or feeding sites more frequently than males without nest boxes indicating that they were socially dominant.
4.1 Mu-opioid receptor densities were lower in POM and VTA in males with compared to without nest boxes
As predicted based on the extreme differences in singing behavior in males with and without nest boxes, as well as past studies implicating opioids in the POM and VTA in male song [5], nest box ownership significantly explained variance in mu-opioid receptor labeling in both POM and VTA. Specifically, male starlings with nest boxes had lower densities of mu-opioid receptors in these regions than males without nest boxes. This finding is similar to a study in rats in which social defeat (similar to a failure to acquire a nest box) resulted in increased mu-opioid receptor mRNA in VTA [29, 30]. In addition to finding mu-opioid receptor optical density to be lower in VTA in males with nest boxes, multiple regression analysis also revealed song to best explain variance in mu-opioid receptor pixel area. This latter finding reflected the fact that males without nest boxes sang at the lowest rates in response to a female and had the greatest area covered by mu-opioid receptor labeling.
In starlings, pharmacological manipulations demonstrate an inhibitory role for opioids in female-directed song [4]. Furthermore, opioids in the avian POM inhibit neuronal firing [31], as well as suppress agonistic, and sexual behaviors in Japanese quail [32]. Given the inhibitory role of opioids in POM, the high densities of mu-opioid receptors in this region in males without nest boxes may make these regions more sensitive to opioid input thereby inhibiting inappropriate social responses to conspecifics. In contrast, the low opioid receptor density in males with nest boxes may render the POM less sensitive to opioids, thereby disinhibiting behavioral responses to female and male conspecifics.
In songbirds, opioids in VTA also act at mu-opioid receptors to inhibit neuronal firing [33]; however unlike opioids in the POM, in rat studies opioids in VTA increase sexually-motivated behavior [34]. This effect is mediated by opioid inhibition of GABA receptors, which causes a disinhibition of activity in VTA dopamine neurons [35, 36]. However, in a recent study elevated firing rates in VTA neurons and dopamine release were found in association with social defeat in male rats [37]. Thus, the finding that males that failed to acquire nest boxes have elevated densities of mu-opioid receptors in VTA may reflect a role for opioid stimulation of dopamine release associated with social defeat.
Previously, in male starlings immunolabeling density for the opioid met-enkephalin in POM correlated positively with undirected but not female-directed song (with a similar trend observed for VTA; p = 0.06) [5]. The limited range of singing observed in males without nest boxes in the present study prevented us from running a similar analysis; however, the present finding that males without nest boxes (i.e., males that sing undirected but not directed song) have elevated densities of mu-opioid receptors in POM and VTA is consistent with the met-enkephalin study and with a study in male zebra finches showing opioid blockade to more potently suppress undirected as opposed to directed song [15]. We have previously proposed that the production of undirected song is intrinsically motivated and reinforced by opioid release in POM and VTA [6]. The present data showing opioids to be elevated in the group of males singing only undirected song are consistent with this hypothesis.
Here, we report a negative correlation between the area covered by mu-opioid receptor pixel area in VTA and song when males with and without nest boxes were combined. This may reflect an inhibitory role for mu receptors in VTA in song in response to a female in males without nest boxes. However it is somewhat in contrast with past data showing labeling for the opioid met-enkephalin to relate positively to male song production when males singing to attract a female in spring and males singing undirected songs in fall were combined [5]. Interpretation of these differences is difficult because met-enkephalin labeling can reflect stored or released enkephalin and because of the dynamic relationships that exist between receptors and peptides (e.g., receptors can be up-regulated in the presence of low levels of opioid release). Thus, the reason that song relates positively to met-enkephalin labeling but negatively to mu-opioid receptor labeling must be examined in future work using direct measures of opioid release or opioid receptor manipulations in the VTA.
4.2 Possible roles for opioids in BSTm and PVN in males with and without nest boxes
The present results also suggest two novel regions in which opioids may regulate song and agonistic interactions, the BSTm and PVN. BSTm is implicated in sexual and agonistic behaviors in birds as in mammals [11, 38–42]. Immediate early gene responses in BSTm differ in male starlings with and without nest boxes and depending on whether males are singing to attract a female or singing undirected song [7, 9]. Across vertebrates, the PVN has been implicated in agonistic behaviors and responses to stressors, including social stressors such as opposite sex conspecific intruders [43–47]. Little is known about the role of opioids in BSTm or PVN in social behavior, however opioids in BSTm can inhibit neuronal firing [48] and male sexual behavior in rodents [49]. Therefore, the elevated mu-opioid receptor activity in BSTm in males without nest boxes may serve to inhibit sexual behavior. Opioids in the PVN have been found to modify physiological responses to stress in rodents [50]. Accordingly, the differences observed in mu-opioid receptors in PVN in the present study may reflect opioid regulation of differential stress responses to social challenges in males with and without nest boxes.
4.3 What do differences in receptor densities reflect?
Reductions in mu-opioid receptor density measures may reflect a decrease in receptor numbers or reduced opioid receptor activation. When bound and activated mu-opioid receptors internalize, which is reflected as an increase in optical density [51–54]. Thus, opioid receptors may be more active in males without nest boxes in POM, VTA, BSTm and PVN. In POM and BSTm the area (pixel area) covered by labeled receptors was also reduced in nest box owners. The differences in pixel area may reflect differences in the sensitivity of a region to opioids; with the larger the area covered by receptors indicating a possible greater sensitivity of the region to opioids.
Interestingly, only nest box ownership (and not agonistic or singing behavior) explained variance in mu receptor measures in POM, PVN, and BSTm. This suggests owning a nest box rather than exhibiting associated behaviors either causes a reduction in opioid receptors in these areas, or conversely that only males with lower densities of mu receptors are motivated to own a nest box. Whether the differences in mu-opioid receptor densities are the cause or consequence of acquiring a nest box is not clear from the present study. This issue must be resolved with studies in which males are assigned individually to aviaries with or without nest boxes or by examining effects of site specific pharmacological manipulations of opioid receptors on behavior of males with and without nest boxes.
4.4 Testosterone and differences in males with and without nest boxes
T is linked to nest box ownership [2, 3, 28], as well as song in response to a female [2, 3, 20]. In the present study males received implants of T, and serum concentrations of T did not differ in males with or without nest boxes. This suggests that differences in behavior and mu-opioid receptors observed in males with and without nest boxes were not determined by differences in serum T, but perhaps by resource possession alone or by the production of behaviors associated with nest box ownership. However, a recent study in territorial California mice (Peromyscus californicus) demonstrates that winning an agonistic interaction (which can be considered similar to “winning” a nest box in starlings) results in increased expression of androgen receptors in BSTm and VTA [55]. Thus, it may be that acquisition of a nest box results in increased sensitivity of neural tissue to androgens which then modifies mu-opioid receptors and behavior (independent of serum T concentrations). This possibility must be addressed in future studies.
4.5 Concluding remarks
The results of the present study suggest that the acquisition of a nest box may cause a reduction in opioid receptor activity within the BSTm, PVN, POM, and VTA of male starlings to promote agonistic responses to males and sexually motivated vocal responses to females. The results are consistent with past studies suggesting a context-dependent role for opioids in birdsong. Moreover, they suggest an important, testosterone-independent, role for opioids in modifying social behavior so that it is appropriate given the resources available to an individual.
Research Highlights
Male starlings with nest boxes displayed agonistic and courtship behaviors
Male starlings without nest boxes displayed little agonistic or courtship behavior
Mu-opioid receptors in several regions were densest in males without nest boxes
Mu- receptors may inhibit responses to conspecifics in males without nest boxes
Differences in males with and without nest boxes were not dependent on testosterone
Acknowledgements
The data presented in this paper are based upon work supported by grants from R01 MH080225 to LVR. We gratefully acknowledge Kate Skogen, and Chris Elliot for animal care taking; Sharon Stevenson for help with tissue processing and Bill Feeny for help with illustrations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gwinner H, Gwinner E, Dittami J. Effects of nestboxes on LH, testosterone, testicular size, and the reproductive behavior of male European starlings in spring. Behav. 1987;103:68–82. [Google Scholar]
- 2.Riters LV, Eens M, Pinxten R, Duffy DL, Balthazart J, Ball GF. Seasonal changes in courtship song and the medial preoptic area in male European starlings (Sturnus vulgaris) Horm Behav. 2000;38:250–261. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- 3.Sartor JJ, Ball GF. Social suppression of song is associated with a reduction in volume of a song-control nucleus in European starlings (Sturnus vulgaris) Behav Neurosci. 2005;119:233–244. doi: 10.1037/0735-7044.119.1.233. [DOI] [PubMed] [Google Scholar]
- 4.Schroeder MB, Riters LV. Pharmacological manipulations of dopamine and opioids have differential effects on sexually motivated song in male European starlings. Physiol Behav. 2006;88:575–584. doi: 10.1016/j.physbeh.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Riters LV, Schroeder MB, Auger CJ, Eens M, Pinxten R, Ball GF. Evidence for opioid involvement in the regulation of song production in male European starlings (Sturnus vulgaris) Behav Neurosci. 2005;119:245–255. doi: 10.1037/0735-7044.119.1.245. [DOI] [PubMed] [Google Scholar]
- 6.Riters LV. Evidence for opioid involvement in the motivation to sing. J Chem Neuroanat. 2010;39:141–150. doi: 10.1016/j.jchemneu.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heimovics SA, Riters LV. Breeding-context-dependent relationships between song and cFOS labeling within social behavior brain regions in male European starlings (Sturnus vulgaris) Horm Behav. 2006;50:726–735. doi: 10.1016/j.yhbeh.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heimovics SA, Riters LV. Immediate early gene activity in song control nuclei and brain areas regulating motivation relates positively to singing behavior during, but not outside of, a breeding context. J Neurobiol. 2005;65:207–224. doi: 10.1002/neu.20181. [DOI] [PubMed] [Google Scholar]
- 9.Heimovics SA, Riters LV. ZENK labeling within social behavior brain regions reveals breeding context-dependent patterns of neural activity associated with song in male European starlings (Sturnus vulgaris) Behav Brain Res. 2007;176:333–343. doi: 10.1016/j.bbr.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newman SW. The medial extended amygdala in male reproductive behavior a node in the mammalian social behavior network. Ann NY Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- 11.Goodson JL. The vertebrate social behavior network: Evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bottjer SW, Alexander G. Localization of Met-Enkephalin and Vasoactive Intestinal Polypeptide in the Brains of Male Zebra Finches (Part 1 of 2) Brain, Behavior and Evolution. 1995;45:153–165. doi: 10.1159/000113547. [DOI] [PubMed] [Google Scholar]
- 13.Woods JK, Deviche P, Corbitt C. Opioid receptor densities analyzed across seasons in the POM and VTA of the dark-eyed junco, Junco hyemalis. J Chem Neuroanat. 2010;40:123–129. doi: 10.1016/j.jchemneu.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Khurshid N, Agarwal V, Iyengar S. Expression of [mu]- and [delta]-opioid receptors in song control regions of adult male zebra finches (Taenopygia guttata) Journal of Chemical Neuroanatomy. 2009;37:158–169. doi: 10.1016/j.jchemneu.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Khurshid N, Jayaprakash N, Hameed LS, Mohanasundaram S, Iyengar S. Opioid modulation of song in male zebra finches (Taenopygia guttata) Behav Brain Res. 2010;208:359–370. doi: 10.1016/j.bbr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Kessel B. Criteria for Sexing and Aging European Starlings (Sturnus vulgaris) Bird-Banding. 1951;22:16–23. [Google Scholar]
- 17.Heimovics SA, Riters LV. Evidence that dopamine within motivation and song control brain regions regulates birdsong context-dependently. Physiol Behav. 2008;95:258–266. doi: 10.1016/j.physbeh.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawson A. Plasma gonadal steroid levels in wild starlings (Sturnus vulgaris) during the annual cycle and in relation to the stages of breeding. Gen Comp Endocr. 1983;49:286–294. doi: 10.1016/0016-6480(83)90146-6. [DOI] [PubMed] [Google Scholar]
- 19.Alger SJ, Riters LV. Lesions to the Medial Preoptic Nucleus Differentially Affect Singing and Nest Box-Directed Behaviors Within and Outside of the Breeding Season in European Starlings (Sturnus vulgaris) Behavioral Neuroscience. 2006;120:1326–1336. doi: 10.1037/0735-7044.120.6.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinxten R, De Ridder E, Balthazart J, Eens M. Context-dependent effects of castration and testosterone treatment on song in male European starlings. Horm Behav. 2002;42:307–318. doi: 10.1006/hbeh.2002.1824. [DOI] [PubMed] [Google Scholar]
- 21.Eens M. Advances in the Study of Behavior. Academic Press; 1997. Understanding the complex song of the European starling: An integrated approach; pp. 355–434. [Google Scholar]
- 22.Hoffman GE, Le WW. Just cool it!: Cryoprotectant anti-freeze in immunocytochemistry and in situ hybridization. Peptides. 2004;25:425–431. doi: 10.1016/j.peptides.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Kerros C, Brood I, Sola B, Jauzac P, Allouche S. Reduction of cell proliferation and potentiation of Fas-induced apoptosis by the selective kappa-opioid receptor agonist U50 488 in the multiple myeloma LP-1 cells. J Neuroim. 2010;220:69–78. doi: 10.1016/j.jneuroim.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Abbadie C, Pan Y-X, Pasternak GW. Differential distribution in rat brain of mu opioid receptor carboxy terminal splice variants MOR-1C-like and MOR-1-like immunoreactivity: Evidence for region-specific processing. J Comp Neurol. 2000;419:244–256. doi: 10.1002/(sici)1096-9861(20000403)419:2<244::aid-cne8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 25.Pan Y-X, Xu J, Mahurter L, Bolan E, Xu M, Pasternak GW. Generation of the mu opioid receptor (MOR-1) protein by three new splice variants of the Oprm gene. P Natl Acad Sci USA. 2001;98:14084–14089. doi: 10.1073/pnas.241296098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garzon J, Juarros JL, Castro MA, Sanchez-Blazquez P. Antibodies to the cloned mu-opioid receptor detect various molecular weight forms in areas of mouse brain. Mol Pharm. 1995;47:738–744. [PubMed] [Google Scholar]
- 27.Goodson JL, Inga DN, Rainer L. Prog Brain Res. Elsevier; 2008. Nonapeptides and the evolutionary patterning of sociality; pp. 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gwinner H, Van't Hof T, Zeman M. Hormonal and behavioral responses of starlings during a confrontation with males or females at nest boxes during the reproductive season. Horm Behav. 2002;42:21–31. doi: 10.1006/hbeh.2002.1795. [DOI] [PubMed] [Google Scholar]
- 29.Nikulina EM, Miczek KA, Hammer RP. Prolonged effects of repeated social defeat stress on mRNA expression and function of [mu]-opioid receptors in the ventral tegmental area of rats. Neuropsychopharmacol. 2005;30:1096–1103. doi: 10.1038/sj.npp.1300658. [DOI] [PubMed] [Google Scholar]
- 30.Nikulina EM, Arrillaga-Romany I, Miczek KA, Hammer RP. Long-lasting alteration in mesocorticolimbic structures after repeated social defeat stress in rats: time course of µ-opioid receptor mRNA and FosB/ΔFosB immunoreactivity. Eur J Neurosci. 2008;27:2272–2284. doi: 10.1111/j.1460-9568.2008.06176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furukawa Y, Kotegawa T, Tsutsui K. Effects of opioid peptides on the electrical activity of preoptic and hypothalamic neurons in the quail brain. J Exp Zool. 1995;273:96–103. doi: 10.1002/jez.1402730203. [DOI] [PubMed] [Google Scholar]
- 32.Kotegawa T, Abe T, Tsutsui K. Inhibitory role of opioid peptides in the regulation of aggressive and sexual behaviors in male japanese quails. J Exp Zool. 1997;277:146–154. doi: 10.1002/(sici)1097-010x(19970201)277:2<146::aid-jez6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 33.Gale SD, Perkel DJ. Physiological properties of zebra finch ventral tegmental area and substantia nigra pars compacta neurons. J Neurophysiol. 2006;96:2295–2306. doi: 10.1152/jn.01040.2005. [DOI] [PubMed] [Google Scholar]
- 34.Van Furth WR, Van Emst MG, Van Ree JM. Opioids and sexual behavior of male rats: Involvement of the medial preoptic area. Behav Neurosci. 1995;109:123–134. doi: 10.1037//0735-7044.109.1.123. [DOI] [PubMed] [Google Scholar]
- 35.Seutin V, Johnson SW, North RA. Apamin increases NMDA-induced burst-firing of rat mesencephalic dopamine neurons. Brain Res. 1993;630:341–344. doi: 10.1016/0006-8993(93)90675-d. [DOI] [PubMed] [Google Scholar]
- 36.Kalivas PW, Churchill L, Klitenick MA. GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neurosci. 1993;57:1047–1060. doi: 10.1016/0306-4522(93)90048-k. [DOI] [PubMed] [Google Scholar]
- 37.Anstrom KK, Miczek KA, Budygin EA. Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neurosci. 2009;161:3–12. doi: 10.1016/j.neuroscience.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bester-Meredith JK, Young LJ, Marler CA. Species differences in paternal behavior and aggression in Peromyscus and their associations with vasopressin immunoreactivity and receptors. Horm Behav. 1999;36:25–38. doi: 10.1006/hbeh.1999.1522. [DOI] [PubMed] [Google Scholar]
- 39.Maney DL, Erwin KL, Goode CT. Neuroendocrine correlates of behavioral polymorphism in white-throated sparrows. Horm Behav. 2005;48:196–206. doi: 10.1016/j.yhbeh.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Goodson JL, Wang Y. Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. P Natl Acad Sci. 2006;103:17013–17017. doi: 10.1073/pnas.0606278103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goodson JL, Rinaldi J, Kelly AM. Vasotocin neurons in the bed nucleus of the stria terminalis preferentially process social information and exhibit properties that dichotomize courting and non-courting phenotypes. Horm Behav. 2009;55:197–202. doi: 10.1016/j.yhbeh.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Z, Smith W, Major DE, De Vries GJ. Sex and species differences in the effects of cohabitation on vasopressin messenger RNA expression in the bed nucleus of the stria terminalis in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) Brain Res. 1994;650:212–218. doi: 10.1016/0006-8993(94)91784-1. [DOI] [PubMed] [Google Scholar]
- 43.Kollack-Walker S, Watson SJ, Akil H. Social stress in hamsters: Defeat activates specific neurocircuits within the brain. J Neurosci. 1997;17:8842–8855. doi: 10.1523/JNEUROSCI.17-22-08842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ebner K, Wotjak CT, Landgraf R, Engelmann M. Neuroendocrine and behavioral response to social confrontation: residents versus intruders, active versus passive coping styles. Hormones and Behavior. 2005;47:14–21. doi: 10.1016/j.yhbeh.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Goodson JL, Evans AK. Neural responses to territorial challenge and nonsocial stress in male song sparrows: segregation, integration, and modulation by a vasopressin V1 antagonist. Horm Behav. 2004;46:371–381. doi: 10.1016/j.yhbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Van de Kar LD, Blair ML. Forebrain pathways mediating stress-induced hormone secretion. Front Neuroendocrin. 1999;20:1–48. doi: 10.1006/frne.1998.0172. [DOI] [PubMed] [Google Scholar]
- 47.Goodson JL, Evans, Andrew K, Soma, Kiran Kb. Neural responses to aggressive challenge correlate with behavior in nonbreeding sparrows. NeuroReport. 2005;16:1719–1723. doi: 10.1097/01.wnr.0000183898.47160.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sawada S, Yamamoto C. Postsynaptic inhibitory actions of catecholamines and opioid peptides in the bed nucleus of the stria terminalis. Exp Brain Res. 1981;41:264–270. doi: 10.1007/BF00238883. [DOI] [PubMed] [Google Scholar]
- 49.Hughes AM, Everitt BJ, Herbert J. Selective effects of [beta]-endorphin infused into the hypothalamus, preoptic area and bed nucleus of the stria terminalis on the sexual and ingestive behaviour of male rats. Neurosci. 1987;23:1063–1073. doi: 10.1016/0306-4522(87)90181-3. [DOI] [PubMed] [Google Scholar]
- 50.Kiritsy-Roy JA, Appel NM, Bobbitt FG, Van Loon GR. Effects of mu-opioid receptor stimulation in the hypothalamic paraventricular nucleus on basal and stress-induced catecholamine secretion and cardiovascular responses. J Pharmacol Exp Ther. 1986;239:814–822. [PubMed] [Google Scholar]
- 51.Sinchak K, Micevych PE. Progesterone blockade of estrogen activation of {mu}-opioid receptors regulates reproductive behavior. J Neurosci. 2001;21:5723–5729. doi: 10.1523/JNEUROSCI.21-15-05723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Micevych PE, Rissman EF, Gustafsson JÅ, Sinchak K. Estrogen receptor-α is required for estrogen-induced µ-opioid receptor internalization. J Neurosci Res. 2003;71:802–810. doi: 10.1002/jnr.10526. [DOI] [PubMed] [Google Scholar]
- 53.Coolen LM, Fitzgerald ME, Yu L, Lehman MN. Activation of [mu] opioid receptors in the medial preoptic area following copulation in male rats. Neurosci. 2004;124:11–21. doi: 10.1016/j.neuroscience.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 54.Micevych PE, Eckersell CB, Brecha N, Holland KL. Estrogen modulation of opioid and cholecystokinin systems in the limbic-hypothalamic circuit. Brain Res Bull. 1997;44:335–343. doi: 10.1016/s0361-9230(97)00212-8. [DOI] [PubMed] [Google Scholar]
- 55.Fuxjager MJ, Forbes-Lorman RM, Coss DJ, Auger CJ, Auger AP, Marler CA. Winning territorial disputes selectively enhances androgen sensitivity in neural pathways related to motivation and social aggression. P Natl Acad Sci. 2010;107:12393–12398. doi: 10.1073/pnas.1001394107. [DOI] [PMC free article] [PubMed] [Google Scholar]