Abstract
Treatment with TNFα inhibitors increases risk of reactivating a latent tuberculosis\infection (LTBI). Therefore screening, prior to therapy with TNFα inhibitors, has been recommended, even in low-endemic areas such as well-developed Western Europe countries. We evaluated interferon-gamma release assay (IGRA), as opposed to tuberculin skin test (TST), for detection of LTBI in refractory inflammatory disease patients prior to the initiation of a first TNFα inhibitor. In addition, we evaluated the impact of impaired cellular immunity on IGRA. Patients starting on TNFα inhibition were screened for LTBI by TST and IGRA (Quantiferon-TB Gold). Data on tuberculosis exposure and Bacillus Calmette–Guérin (BCG) vaccination were obtained. Cellular immunity was assessed by CD4+ T lymphocyte cell count. Nine out of 56 patients (16.1%) tested positive for LTBI. A concordant positive result was present in three patients with a medical history of tuberculosis exposure. Six patients with discordant test results had either: (1) a negative TST and positive IGRA in combination with a medical history of tuberculosis exposure (n = 1) or (2) a positive TST and negative IGRA in combination with BCG vaccination (n = 3) or a medical history of tuberculosis exposure (n = 2). CD4+ T lymphocyte cell counts were within normal limits, and no indeterminate results of IGRA were present. IGRA appears reliable for confirming TST and excluding a false positive TST (due to prior BCG vaccination) in this Dutch serie of patients. In addition, IGRA may detect one additional case of LTBI out of 56 patients that would otherwise be missed using solely TST. Immune suppression appears not to result significantly in lower CD4+ T lymphocyte cell counts and indeterminate results of IGRA, despite systemic corticosteroid treatment in half of the patients. Confirmation in larger studies, including assessment of cost-effectiveness, is required.
Keywords: CD4+ T lymphocyte cell count, IGRA, Immune-mediated inflammatory disease, Latent tuberculosis infection, TNFα inhibition, TST
Introduction
Tumor necrosis factor α (TNFα) is a regulating cytokine with a central role in the pathogenesis of chronic inflammatory disease and thereby a well-defined target for intervention. In concordance with this, inhibitors of TNFα have become increasingly important in treatment of a broad spectrum of rheumatic diseases such as rheumatoid arthritis [1], psoriatic arthritis [2], ankylosing spondylitis [3], juvenile inflammatory arthritis [4], adult onset Still’s disease [5], and sarcoidosis [6]. However, TNFα is also an essential component of host defense against pathogenic viruses, bacteria, and fungi, and therapeutic inhibition of TNFα may elicit risk of opportunistic infections [7, 8], in particular, tuberculosis [9, 10]. Thus, screening for LTBI before TNFα inhibition has been recommended, however, no gold standard for detecting LTBI exists today and guidelines have provided conflicting recommendations about the place of diagnostic screening tests such as tuberculin skin test (TST) and interferon-gamma release assay (IGRA).
TST has several limitations as a diagnostic test in detecting LTBI. Firstly, TST attempts to measure cell-mediated immunity by delayed-type hypersensitivity response to purified protein derivate (PPD)—i.e., a crude mixture of mycobacteria antigens. This results in false positive results in non-tuberculosis mycobacterium infection and clinically more important, in Bacillus Calmette–Guérin (BCG)-vaccinated persons [11, 12]. Secondly, TST sensitivity is lower in immunocompromised patients, possibly due to impaired T cell function and impaired cellular immunity [13]. And thirdly, TST has practical disadvantages such as inconvenience (two patient visits) and interobserver variability [14]. With respect to these limitations, an in vitro T cell-based assay has been developed, detecting interferon-gamma in response to contact with antigens highly specific for tuberculosis mycobacteria (ESAT-6, CFP-10, and TB 7.7). This IGRA is not influenced by contact with non-tuberculosis mycobacteria or prior vaccination with BCG [15, 16]. Moreover, it is suggested that IGRA has higher sensitivity in comparison to TST in patients receiving immunosuppressive treatment [13, 17, 18]. In summary, although some evidence exists that IGRA has a better performance in screening of LTBI before starting TNFα inhibition, the true value of IGRA as a diagnostic tool, with respect to TST, is ill-defined.
The objective of this study was to compare TST and IGRA (Quantiferon-TB Gold) in detecting LTBI in refractory inflammatory disease patients prior to the initiation of a first TNFα inhibitor. In addition, we evaluated the impact of cellular immunity on IGRA.
Materials and methods
Between 2008 and 2009, we prospectively enrolled patients with chronic immune-mediated inflammatory diseases starting on TNFα inhibition. Patients were recruited from the rheumatology outpatient clinic of the Medical Center of Leeuwarden, The Netherlands. Patients with rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and juvenile idiopathic arthritis (fulfilling American College of Rheumatology criteria), and two patients with sarcoidosis and Still’s disease refractory to treatment with corticosteroids and methotrexate, were included. The decision to start TNFα inhibition was made in agreement with criteria of the Dutch Society of Rheumatology [19]. The following variables were collected: gender, age, diagnosis, disease duration, and immunosuppressive treatment (type and dosage) at study inclusion. Tuberculosis exposure was assessed by personal and family medical history, previous treatment with tuberculostatic, and BCG vaccination. Chest radiographs were negative for (active) tuberculosis infection in all patients. Patients were tested for LTBI by TST and IGRA (Quantiferon-TB Gold). TNFα inhibition was initiated after a concordant negative TST and IGRA. Patients with discordant or concordant positive test results received adequate tuberculostatic treatment before initiation of TNFα inhibition except for BCG-vaccinated patients with positive TST, negative IGRA, and negative medical history.
TST was performed by intradermal injection of 0.1 ml of PPD at the flexor surface of the upper one third of the forearm according to the Mantoux method. The diameter of skin induration was assessed 48–72 h after inoculation by a trained rheumatologic nurse. A positive TST was defined as ≥5 mm induration according to the Dutch guidelines [19]. Immediately after the intradermal injection of PPD, a heparinized whole blood sample was collected and transferred to three QuantiferonR-TB gold test tubes (Cellestis Ltd, Australia) to prohibit a booster effect. IGRA was performed and interpreted according to instructions of the manufacturer and guideline of Centers of Disease Control and Prevention [20], respectively. An EDTA tube was collected for assessment of cellular immunity by CD4+ cell T lymphocyte count (CD4+ cell count).
Results
Patient characteristics
Twenty-nine out of 56 included patients were diagnosed with rheumatoid arthritis (51.8%) of whom 83.3% were rheumatoid factor positive (Table 1). Other diagnoses were ankylosing spondylitis (30.4%), psoriatic arthritis (8.9%), undifferentiated spondylarthropathy (3.6%), juvenile idiopathic arthritis (1.8%), adult onset Still’s disease (1.8%), and sarcoidosis (1.8%). Mean (± SD) age of the total study population was 50.3 ± 15.4 years, and disease duration was 8.7 ± 9.2 years. About half of the study population (48.2%) had received methotrexate (± corticosteroids), 5.4% leflunomide (± methotrexate ± corticosteroids), 1.8% azathioprine and corticosteroids, 5% only corticosteroids, and 35.7% did not had previous immunosuppressive treatment. Dosage of immunosuppressive treatment is listed in Table 1. The incidence of BCG vaccination in the total investigated population was 5% which is representative of a post-World War II population. Tuberculosis exposure was 14% and is in line with the low prevalence of tuberculosis in Western Europe countries. The absence of reactivation of tuberculosis during TNFα inhibition in the BCG-vaccinated patients ruled out the coexistence of BCG vaccination and prior tuberculosis exposure in this population.
Table 1.
Patient characteristics of 56 patients
| Patient characteristics | No. (%) |
|---|---|
| Male | 32 (53.6%) |
| Age (mean ± SD) | 50.3 ± 15.4 years |
| Diagnosis | |
| Rheumatoid arthritisa | 29 (51.8%) |
| RF positive | 25 (83.3%) |
| Ankylosing spondylitisa | 17 (30.4%) |
| Psoriatic arthritisa | 5 (8.9%) |
| Undifferentiated spondylitis | 2 (3.6%) |
| Juvenile idiopathic arthritisa | 1 (1.8%) |
| Still’s disease | 1 (1.8%) |
| Sarcoidosis | 1 (1.8%) |
| Disease duration (mean ± SD) | |
| Total study population | 8.7 ± 9.2 years |
| Rheumatoid arthritis | 7.4 ± 7.7 years |
| Type of immunosuppressive treatment (at study inclusion) | |
| Methotrexate ± corticosteroids | 27 (48.2%) |
| Leflunomide ± methotrexate ± corticosteroids | 3 (5.4%) |
| Azathioprine and corticosteroids | 1 (1.8%) |
| Only corticosteroids (maintenance and high-dose treatment) | 5 (8.9%) |
| No current immunosuppressive treatment | 20 (35.7%) |
| Daily dose of immunosuppressive treatment (at study inclusion; mean ± SD) | |
| Methotrexate | 19 ± 7 mg |
| Leflunomide | 20 ± 0 mg |
| Azathioprine | 150 mg |
| Corticosteroids | |
| Maintenance treatment | 6 ± 2 mg |
| High-dose treatmentb | 3,000 ± 0 mg |
| BCG vaccination | 3 (5%) |
| Tuberculosis exposure | 8 (14%) |
Data are presented as number (No.) and percentage (%) and, if applicable, mean ± standard deviation (SD)
No. number of patients, RF rheumatoid factor
aDiagnosis according to the American College of Rheumatology criteria
bOnce-only three pulses of 1 g methylprednisolone prior to LTBI screening (n = 3)
Comparison of TST and IGRA
Nine out of 56 patients (16.1%) tested positive for LTBI with either TST or IGRA (Table 2). A concordant positive result was present in three patients with a medical history of tuberculosis exposure. In six patients, discordant results were obtained: one patient had a negative TST and positive IGRA, and five patients had a positive TST and negative IGRA. The mother of the patient with negative TST and positive IGRA had suffered from tuberculosis during his childhood. The five patients with positive TST and negative IGRA were either BCG-vaccinated (three patients) or had a medical history of tuberculosis exposure (two patients). The BCG-vaccinated patients with positive TST and negative IGRA did not develop tuberculosis during TNFα inhibition in the follow-up period (1–2 years). Interestingly, there were no indeterminate results of IGRA.
Table 2.
Characteristics of nine patients with concordant positive TST and IGRA and discordant TST and IGRA
| N | TST | IGRA | BCG | Tuberculosis exposure | CD4+ count (cells/mm3) | Medication (daily dose in mg) | Diagnosis |
|---|---|---|---|---|---|---|---|
| Concordant positive results of TST and IGRA | |||||||
| 1 | pos. | pos. | no | yes | 748 | MTX 12.5; lef 20 | PsA |
| 2 | pos. | pos. | no | yes | 972 | MTX 25 | RA |
| 3 | pos. | pos. | no | yes | N.A. | none | AS |
| Discordant results of TST and IGRA | |||||||
| 1 | neg. | pos. | no | yes | 974 | MTX 15 | RA |
| 2 | pos. | neg. | yes | no | 590 | pred 5; MTX 10; lef 20 | RA |
| 3 | pos. | neg. | yes | no | 387 | MTX 25 | RA |
| 4 | pos. | neg. | yes | no | 2,050 | MTX 7.5 | PsA |
| 5 | pos. | neg. | no | yes | 632 | none | AS |
| 6 | pos. | neg. | no | yes | 549 | MTX 7.5 | RA |
N patient number, TST tuberculosis skin test, IGRA interferon-gamma release assay (Quantiferon-TB Gold), BCG Bacillus Calmette–Guérin, TB tuberculosis, CD4 + count CD4+ T lymphocyte cell count, pos. positive, neg. negative, N.A. not available, MTX methotrexate, pred prednisone, lef leflunomide, PsA psoriatic arthritis, RA rheumatoid arthritis, AS ankylosing spondylitis
Cellular immunity
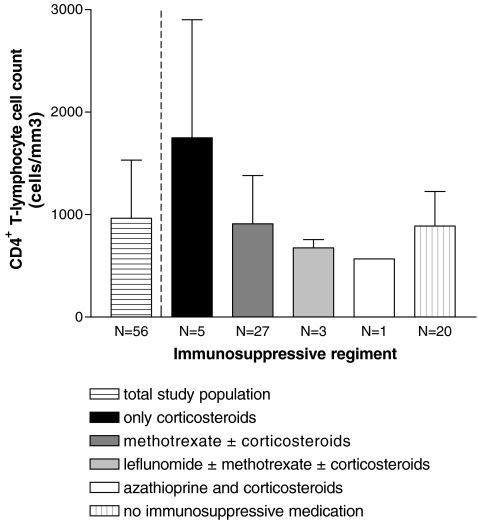
CD4+ cell counts were within normal limits with a mean (± SD) of 964 ± 568 cells/mm3 (Fig. 1). A CD4+ cell count below 300 cells/mm3 was present in two patients receiving methotrexate and low-dose corticosteroids. These two patients had a negative result for TST and IGRA and did not develop tuberculosis during TNFα inhibition (2 years of follow-up). There were no patients with a CD4+ cell count below 200 cells/mm3.
Fig. 1.
Cellular immunity defined by immunosuppressive treatment and CD4+ T lymphocyte cell count (cells/mm3) at study inclusion. Data are given as mean ± standard deviation (SD)
Discussion
The objective of this study was to compare TST and IGRA (Quantiferon-TB Gold) in detecting LTBI in refractory inflammatory disease patients prior to the initiation of a first TNFα inhibitor. A well-recognized problem in screening for LTBI is absence of a gold standard and thereby sensitivity and specificity of TST and IGRA cannot be directly measured. Nevertheless, assessment of tuberculosis exposure, combined with results of TST and IGRA, may roughly estimate the a priori chance of LTBI.
Nine out of 56 patients (16.1%) tested positive for LTBI with either TST or IGRA. A concordant positive result was present in three patients with a medical history of tuberculosis exposure. The remaining six patients with discordance had either a negative TST and positive IGRA (one patient) or a positive TST and negative IGRA (five patients). The discordance in the five patients with positive TST and negative IGRA can be attributed to BCG vaccination (three patients) or a medical history of tuberculosis exposure (two patients). As for the patients with negative IGRA and positive TST, it cannot be excluded that IGRA may be false negative as the infection occurred in the distant past. This may be explained by the fact that IGRA mostly measures effector T cell responses whereas TST measures both effector and memory T cell responses. After 24 h incubation in the IGRA, only circulating effector memory T cells have sufficient time to produce interferon, while central memory T cells first started producing interferon after a more prolonged (72 h in TST) incubation [21]. The Quantiferon-TB Gold performs well in routine screening of low-prevalence populations, but its performance turned out to be suboptimal in healthy persons with a high risk of tuberculosis exposure [22]. It is also known that the sensitivity of the Quantiferon depends on the test and is higher for the latest in-tube version that was used in this study [23]. Indeed, we found a low number (n = 1) of discordant negative TST and positive IGRA in a low tuberculosis-exposed population.
Indeterminate results of IGRA are commonly reported in patients with, e.g., HIV, malignancy, and chronic renal failure, and patients undergoing immunosuppressive treatment [24, 25]. In comparison with studies in rheumatic disease patients [26–32], it is remarkable that in this study IGRA could be interpreted without problems—i.e., there were no indeterminate results. This suggests that lymphocytes retained the capacity to produce interferon-gamma on mitogen stimulation in vitro, even with immunosuppressive treatment. Although we do not have a representative control group, it appears that CD4+ cell count in this study population was not greatly reduced in response to immunosuppressive treatment—i.e., CD4+ cell counts were not below 200 cells/mm3 and only two patients had a CD4+ cell count below 300 cells/mm3. Furthermore, the two patients with a positive TST, a negative IGRA and an positive medical history of LTBI had both a normal CD4+ cell count.
The absence of a significant influence of immunosuppressive treatment on interpretation of IGRA in patients with inflammatory rheumatic conditions has previously been reported by Matulis [27]. Cellestis stated that as long as the viable CD4+ cell count is above 200 cells/mm3 Quantiferon-TB Gold has a good performance. However, it has been questioned whether CD4+ cell count can be used as a marker of validity of IGRA in patients other than HIV patients [28]. Furthermore, the intrinsic function of T cells may be an important precondition for the capacity of interferon production. Two studies analyzing the impact of different classes of drugs on the response of TST and IGRA in European patients with immune-mediated inflammatory diseases stated that corticosteroid treatment can in fact compromise test results by increasing the number of indeterminate results of IGRA and negative TST outcomes [27, 29]. We therefore hypothesize that high-dose corticosteroid maintenance therapy may be a risk factor for an indeterminate IGRA result. Possibly, the absence of indeterminate test IGRA results in our study was due to the low-dose corticosteroids (10 mg per day at most). Overall, sensitivity of IGRA for the diagnosis of LTBI might be higher than TST, despite a higher rate of indeterminate results in immunocompromised patients [26–32].
Various recommendations and guidelines have been issued to determine the place of diagnostic screening test for LTBI. They are traditionally based on medical history, chest X-ray and TST. Two leading institutes, the Centers of Disease Control and Prevention (CDC) of the United States and United Kingdom’s National Institute for health and Clinical Excellence (NICE), have developed guidelines regarding correct usage of both diagnostic tools [20, 33], but they came to conflicting conclusions. The CDC stated that IGRA is a real alternative for TST whereas NICE advised the use of IGRA only for the validation of a positive TST. The variation in prevalence of tuberculosis over time in different parts of the world may account for this disagreement. Western Europe has a substantially larger BCG-vaccinated population as compared to the United States of America (USA). Therefore, the addition of IGRA augments the specificity of prescreening in Western Europe. Furthermore, most cases of LBTI in Western Europe occurred in a population born before 1945. A lower performance of IGRA in Europe as compared to the USA may be attributed to a low sensitivity in the case of longer duration of LTBI. In different countries of Western Europe IGRA is currently added in a second step approach according to NICE or is even replacing TST (Germany, Switzerland) [34, 35]. We feel that all physicians, including rheumatologists, who treat different immune-mediated inflammatory diseases, are obliged to hand tailor the prophylactic therapy for LTBI by the prevalence of tuberculosis and BCG vaccination of their country.
In conclusion, the findings of this study suggest that IGRA appears reliable for confirming a positive TST and excluding a false positive result (in case of prior BCG vaccination) in this Dutch serie of patients. IGRA may thereby reduce the number of patients in whom tuberculostatics are prescribed in absence of LTBI, resulting in an evident benefit of avoiding a 6-month delay of effective anti-rheumatic treatment with TNFα inhibition and possible side effects due to treatment with tuberculostatics. Moreover, IGRA seems to detect an additional case of LTBI that otherwise be missed using solely TST. IGRA appears not to be significantly influenced by immunosuppressive treatment, at least not by the regimen and dosage used in this study. Although the number of patients is not large enough for powerful statistical analysis and patient characteristics are quite heterogeneous, this study contributes to the ongoing discussion on the diagnostic value of IGRA. IGRA should not be employed as an alternative single screening test as false negative results have been observed. Confirmation in larger studies, including cost-effectiveness in different countries to determine the optimal screening strategy, is warranted.
Acknowledgments
Disclosures
None
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370:1861–1874. doi: 10.1016/S0140-6736(07)60784-3. [DOI] [PubMed] [Google Scholar]
- 2.Mease PJ. Psoriatic arthritis assessment and treatment update. Curr Opin Rheumatol. 2009;21:348–355. doi: 10.1097/BOR.0b013e32832c7832. [DOI] [PubMed] [Google Scholar]
- 3.Braun J, Davis J, Dougados M, Sieper J, van der Linden S, van der Heijde D. First update of the international ASAS consensus statement for the use of anti-TNF agents in patients with ankylosing spondylitis. Ann Rheum Dis. 2006;65:316–320. doi: 10.1136/ard.2005.040758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quartier P, Taupin P, Bourdeaut F, et al. Efficacy of etanercept for the treatment of juvenile idiopathic arthritis according to the onset type. Arthritis Rheum. 2003;48:1093–1101. doi: 10.1002/art.10885. [DOI] [PubMed] [Google Scholar]
- 5.Kraetsch HG, Antoni C, Kalden JR, Manger B. Successful treatment of a small cohort of patients with adult onset of Still's disease with infliximab: first experiences. Ann Rheum Dis. 2001;60(Suppl 3):iii55–iii57. doi: 10.1136/ard.60.90003.iii55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saleh S, Ghodsian S, Yakimova V, Henderson J, Sharma OP. Effectiveness of infliximab in treating selected patients with sarcoidosis. Respir Med. 2006;100:2053–2059. doi: 10.1016/j.rmed.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 7.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 8.Patkar NM, Teng GG, Curtis JR, Saag KG. Association of infections and tuberculosis with antitumor necrosis factor alpha therapy. Curr Opin Rheumatol. 2008;20:320–326. doi: 10.1097/BOR.0b013e3282fa74f7. [DOI] [PubMed] [Google Scholar]
- 9.Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 10.Gomez-Reino JJ, Carmona L, Angel Descalzo M. Risk of tuberculosis in patients treated with tumor necrosis factor antagonists due to incomplete prevention of reactivation of latent infection. Arthritis Rheum. 2007;57:756–761. doi: 10.1002/art.22768. [DOI] [PubMed] [Google Scholar]
- 11.Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet. 2000;356:1099–1104. doi: 10.1016/S0140-6736(00)02742-2. [DOI] [PubMed] [Google Scholar]
- 12.Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med 2000; 161:1376–1395 [DOI] [PubMed]
- 13.Chen DY, Shen GH, Hsieh TY, Hsieh CW, Lan JL. Effectiveness of the combination of a whole-blood interferon-gamma assay and the tuberculin skin test in detecting latent tuberculosis infection in rheumatoid arthritis patients receiving adalimumab therapy. Arthritis Rheum. 2008;59:800–806. doi: 10.1002/art.23705. [DOI] [PubMed] [Google Scholar]
- 14.Pouchot J, Grasland A, Collet C, Coste J, Esdaile JM, Vinceneux P. Reliability of tuberculin skin test measurement. Ann Intern Med. 1997;126:210–214. doi: 10.7326/0003-4819-126-3-199702010-00005. [DOI] [PubMed] [Google Scholar]
- 15.Brock I, Weldingh K, Lillebaek T, Follmann F, Andersen P. Comparison of tuberculin skin test and new specific blood test in tuberculosis contacts. Am J Respir Crit Care Med. 2004;170:65–69. doi: 10.1164/rccm.200402-232OC. [DOI] [PubMed] [Google Scholar]
- 16.Kang YA, Lee HW, Yoon HI, et al. Discrepancy between the tuberculin skin test and the whole-blood interferon gamma assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA. 2005;293:2756–2761. doi: 10.1001/jama.293.22.2756. [DOI] [PubMed] [Google Scholar]
- 17.Pai M, Riley LW, Colford JM., Jr Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis. 2004;4:761–776. doi: 10.1016/S1473-3099(04)01206-X. [DOI] [PubMed] [Google Scholar]
- 18.Lalvani A. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest. 2007;131(6):1898–1906. doi: 10.1378/chest.06-2471. [DOI] [PubMed] [Google Scholar]
- 19.Dutch Society of Rheumatology (2003) Tuberculosis and TNFa-blockade (guideline)
- 20.Mazurek GH, Villarino ME. Guidelines for using the QuantiFERON-TB test for diagnosing latent mycobacterium tuberculosis infection. Centers for disease control and prevention. MMWR Recomm Rep. 2003;31:15–18. [PubMed] [Google Scholar]
- 21.Leyten EM, Arend SM, Prins C, Cobelens FG, Ottenhoff TH, van Dissel JT. Discrepancy between mycobacterium tuberculosis-specific gamma interferon release assays using short and prolonged in vitro incubation. Clin Vaccine Immunol. 2007;14:880–885. doi: 10.1128/CVI.00132-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Neal S, Hedberg K, Markum A, Schafer S. Discordant tuberculin skin and interferon-gamma tests during contact investigations: a dilemma for tuberculosis controllers. Int J Tuberc Lung Dis. 2009;13(5):662–664. [PubMed] [Google Scholar]
- 23.Saracino A, Scotto G, Fornabaio C, et al. Quantiferon-TB Gold in-tube test (QFT-GIT) for the screening of latent tuberculosis in recent immigrants in Italy. New Microbiol. 2009;32:369–376. [PubMed] [Google Scholar]
- 24.Kobashi Y, Obase Y, Fukuda M, Miyashita N, Oka M. Clinical evaluation of QuantiFERON TB-2G test for immunocompromised patients. Eur Respir J. 2007;30:945–950. doi: 10.1183/09031936.00040007. [DOI] [PubMed] [Google Scholar]
- 25.Richeldi L, Losi M, D’Amico R, et al. Performance of tests for latent tuberculosis in different groups of immunocompromised patients. Chest. 2010;136:198–204. doi: 10.1378/chest.08-2575. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg JD, Reddy SM, Schloss SG, et al. Comparison of an in vitro tuberculosis interferon-gamma assay with delayed-type hypersensitivity testing for detection of latent mycobacterium tuberculosis: a pilot study in rheumatoid arthritis. J Rheumatol. 2008;35:770–775. [PubMed] [Google Scholar]
- 27.Matulis G, Juni P, Villiger PM, Gadola SD. Detection of latent tuberculosis in immunosuppressed patients with autoimmune diseases: performance of a mycobacterium tuberculosis antigen-specific interferon gamma assay. Ann Rheum Dis. 2008;67:84–90. doi: 10.1136/ard.2007.070789. [DOI] [PubMed] [Google Scholar]
- 28.Soborg B, Ruhwald M, Hetland ML, et al. Comparison of screening procedures for mycobacterium tuberculosis infection among patients with inflammatory diseases. J Rheumatol. 2009;36:1876–1884. doi: 10.3899/jrheum.081292. [DOI] [PubMed] [Google Scholar]
- 29.Inanc N, Aydin SZ, Karakurt S, Atagunduz P, Yavuz S, Direskeneli H. Agreement between Quantiferon-TB Gold test and tuberculin skin test in the identification of latent tuberculosis infection in patients with rheumatoid arthritis and ankylosing spondylitis. J Rheumatol. 2009;36:2675–2681. doi: 10.3899/jrheum.090268. [DOI] [PubMed] [Google Scholar]
- 30.Bartalesi F, Vicidomini S, Goletti D, et al. Quantiferon-TB Gold and the TST are both useful for latent tuberculosis infection screening in autoimmune diseases. Eur Respir J. 2009;33:586–593. doi: 10.1183/09031936.00107608. [DOI] [PubMed] [Google Scholar]
- 31.Kim EY, Jung JY, Son JY, et al. Performance of the tuberculin skin test and interferon-gamma release assay for detection of tuberculosis infection in immunocompromised patients in a BCG-vaccinated population. BMC Infect Dis. 2009;15:9–207. doi: 10.1186/1471-2334-9-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin J, Walsh C, Gibbs A, et al. Comparison of interferon-{gamma}-release assays and conventional screening tests before tumour necrosis factor-{alpha} blockade in patients with inflammatory arthritis. Ann Rheum Dis. 2010;69:181–185. doi: 10.1136/ard.2008.101857. [DOI] [PubMed] [Google Scholar]
- 33.British National Institute for Health and Clinical Excellence (2006). Tuberculosis; clinical diagnosis and management of tuberculosis and measures for its prevention and control (guideline). London, UK:NICE:2006. http://www.nice.org.uk/guidance/CG33. Accessed March 2009 [PubMed]
- 34.Beglinger C, Dudler J, Mottet C, et al. Screening for tuberculosis infection before the initiation of an anti-TNF-alpha therapy. Swiss Med Wkly. 2007;137:620–622. doi: 10.4414/smw.2007.11939. [DOI] [PubMed] [Google Scholar]
- 35.Diel R, Hauer B, Loddenkemper R, Manger B, Kruger K. Recommendations for tuberculosis screening before initiation of TNF-alpha-inhibitor treatment in rheumatic diseases. Pneumologie. 2009;63:329–334. doi: 10.1055/s-0029-1214673. [DOI] [PubMed] [Google Scholar]