Abstract
Imaging recognition of multidrug-resistance by 99mTc-labeled sestamibi, tetrofosmin and furifosmin in mice bearing human breast tumors was evaluated using a high-resolution SPECT, FASTSPECT. Imaging results showed that the washout rates in drug-resistant MCF7/D40 tumors were significantly greater than that in drug-sensitive MCF7/S tumors. Furifosmin exhibited greater washout from both MCF7/S and MCF7/D40 than sestamibi, while tetrofosmin washout was greater than sestamibi in MCF7/D40 only. Feasibility of the monocationic agents for characterizing MDR expression was well clarified with FASTSPECT imaging.
Keywords: High-resolution SPECT, 99mTc-tetrofosmin, 99mTc-sestamibi, 99mTc-furifosmin, Multidrug resistance, Breast tumor
1. Introduction
Breast cancer ranks second among cancer deaths in women. Doxorubicin is one of the most effective chemotherapeutic agents available for patients with breast cancer. However, the effectiveness of doxorubicin and other chemotherapeutic drugs can be hindered by the resistance of malignant tumors to chemotherapy [1–3], resulting in treatment failure. The multidrug resistance (MDR) gene (MDR1), a gene that encodes a transmembrane glycoprotein, P-glycoprotein (Pgp), is the best-characterized mechanism of resistance to many drugs. Pgp is an energy-dependent drug-efflux pump [4–8]. It has been found that Pgp, as an efflux pump, transports out of tumor cells a wide range of structurally and functionally unrelated cytotoxic drugs including doxorubicin, etoposide, paclitaxel, vincristine and many others. Along with the drugs, many surrogate markers of Pgp function in vivo such as 99mTc-labeled radiopharmaceuticals can also be pumped out of cells with MDR expression [9–16].
99mTechnetium (99mTc) labeled sestamibi (MIBI), tetrofosmin and furifosmin (Q-12) are lipophilic monocationic radiotracers that were originally designed for imaging myocardial perfusion [17–18]. Sestamibi (Cardiolite®) is an isonitrile, while tetrofosmin (Myoview™) and furifosmin are phosphines. These agents share the properties of lipophilicity, small molecular size and monocationic charge. Cellular uptake of the cationic agents is, in large measure, driven by electrical transmembrane potential differences. They have been proposed as transport substrates for Pgp and tested in a variety of MDR tumor cells [9–16]. It has been suggested that cellular accumulation of the cationic agents is inversely proportional to the level of Pgp expression, and enhancement of radioactivity in tumor cells is observed after exposure to MDR modulators or chemosensitizers. These MDR modulators inhibit Pgp function and increase intracellular concentrations of chemotherapeutic drugs in tumors; examples of modulators include verapamil, cyclosporin A, GG918 (elacridar) and PSC833 (valspodar) [15,19–23].
To date, most of the available results of the three lipophilic cationic agents in detecting MDR in tumors are from in vitro studies. No quantitative data have been reported that compare the monocationic agents directly in the same breast-cancer animal model by in vivo imaging. In order to quantify radiopharmaceutical kinetics in vivo in small animals with size-limited tumor xenografts, a high-resolution SPECT camera is more useful than a pinhole planar gamma camera. The Radiology Research Laboratory at the University of Arizona has designed and built a high-resolution stationary SPECT camera system called FASTSPECT [24–26]. This system consists of twenty-four modular cameras with a multiple-pinhole aperture. FASTSPECT is especially useful for small animal imaging because of its high spatial resolution. The purpose of this study was to compare the in vivo properties of 99mTc-sestamibi, 99mTc-tetrofosmin and 99mTc-furifosmin in identifying MDR using FASTSPECT imaging; this was done in well-defined severe combined immunodeficient (SCID) mouse models with human breast-cancer xenografts. We wanted to clarify whether FASTSPECT can provide fast dynamic acquisition of tomographic images in the mice, as well as quantify uptake and clearance kinetics of 99mTc-labeled monocationic agents.
2. Materials and methods
2.1. Tumor cell lines
MCF7/S cell lines: Cells are parental, drug (doxorubicin) sensitive, breast carcinoma cells that were originally obtained from American Type Culture Collection (ATCC #HTB-22, Rockville, MD).
MCF7/D40 Cell lines: Cells are generated in vitro by successive culturing parental MCF7 cells in slowly increasing concentrations of doxorubicin in a multiple step procedure with a total selection time of 31 months [27]. The initial concentration of doxorubicin was 1 × 10−8M. Fresh drug was added when the medium was changed three times a week. Over a period of 19 months the concentration of doxorubicin was increased from 1 × 10−8M to 7 × 10−8M. It took an additional 12 months to reach the final concentration of 4 × 10−7M representing a 40-fold increase and full development of the doxorubicin-resistant variant cells. MCF7/D40 are Pgp positive. The presence of Pgp was detected by immunoblot analysis using the C219 mouse monoclonal antibody (IgG). 125I-labeled rabbit anti-mouse IgG as the secondary antibody. The cells were maintained in a drug-free medium for one week prior to experiments.
2.2. Establishment of tumor models
Severe Combined Immunodeficient (SCID) mice weighing 18–22 g were obtained from the SCID mouse core facility at the University of Arizona Comprehensive Cancer Center. Mice were housed under pathogen-free conditions in microisolator cages with laboratory chow and water available.
Tumor cells were grown in RPMI-1640 medium (Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum, 1% L-glutamine and 1% penicillin streptomycin and maintained in a humidified atmosphere of air containing 5% CO2 at 37° C. When the cell cultures were grown to 95%–100% confluence, trypsin (1 ml trypsin solution/5 ml of Hanks balanced salt solution, HBSS) was added to detach adherent cells. The cells (90–100% viability) were counted and re-suspended at a concentration of 9 × 106 cells in sterile saline. Subcutaneous breast adenocarcinomas were established by injecting 9 × 106 MCF7/S or MCF7/D40 cells in a total volume of 200 μL into the SCID mouse right thigh. The volume of tumor was monitored. After 10 to 14 days, tumors reached a size of 200–500 mm3, appropriate for imaging.
On the day of imaging, animals were anesthetized with (1.0%–1.5%) isoflurane. The jugular vein was catheterized with a PE-10 catheter using the following surgical procedure. The ventral cervical area was clipped and prepared. An incision was made over the jugular furrow, and blunt and sharp dissection were used to isolate the jugular vein. The vein was ligated rostrally with 4-0 suture. A nick was made in the vein using micro scissors, and the catheter was advanced through the opening and secured proximal to the opening with a ligature, and further secured distal to the opening with a second ligature. A simple continuous pattern with 3-0 suture was used for closure.
2.3. Experimental groups
MCF7/S tumor model: A total of 17 SCID mice with MCD7/S tumors were studied. FASTSPECT imaging was performed in 5 mice with 99mTc-sestamibi, 5 with 99mTc-tetrofosmin and 7 with 99mTc-furifosmin.
MCF7/D40 tumor model: There were 21 SCID mice with MCF7/D40 tumors imaged with FASTSPECT. The images were obtained in 9 mice with 99mTc-sestamibi, 6 with 99mTc-tetrofosmin and 6 with 99mTc-furifosmin.
2.4. Radiopharmaceutical preparation
99mTc-sestamibi was prepared with a Cardiolite® kit (Bristol-Myers Squibb) provided by Syncor Corporation. The radiochemical purity (RCP) was greater than 95%. Tetrofosmin (Myoview™) kits were gifts from Amersham Health. Tetrofosmin was radiolabeled according to the manufacturer’s instructions. One ml of sodium pertechnetate-99m (no less than 70 mCi, 2.59 GBq) was added to the vial and allowed to react at room temperature for 15 min. 99mTc-tetrofosmin RCP was more than 95% as determined by chromatography with ITLC SG 10 cm × 1 cm strip and 35:65 acetone:dichloromethane solvent. Furifosmin kits were gifts from Mallinckrodt Medical. A vial of furifosmin was injected with 1.5 ml sterile sodium pertechnetate solution containing 70 mCi (2.59 GBq) 99mTc. The vial was placed in a boiling water bath for 20 minutes and then cooled to room temperature. The RCP of 99mTc-furifosmin complexes was determined using Sep-Pak R Alumina cartridges (Millipore Corp., Milford, MA). A 0.1 ml sample of 99mTc-furifosmin was injected into the long end of the cartridge. Ten ml 200 proof ethanol were injected into the cartridge and the elution was collected. Ten ml saline were injected next followed by 10 ml air, and the elution from these two steps was collected separately from the ethanol-based elution. The activities of each of the two elutions and the cartridge were measured separately. The first elution contained pure 99mTc-furifosmin. The second elution and the cartridge contained impurities. Purity of the sample was calculated by dividing the activity of the first elution by the sum of the activities of the second elution and the cartridge. The RCP values ranged from 90% to 95%.
2.5. High-resolution spect imaging acquisition
High-resolution images were obtained using the stationary SPECT system, FASTSPECT, built in the Radiology Research Laboratory at the University of Arizona. It consists of 24 10 cm × 10 cm modular gamma cameras and a cylindrical aperture. Twenty-four 1 mm diameter pinholes were drilled in the aperture such that a point source in the center of the field of view is simultaneously projected to the center of each camera. The total magnification is 3.5 in a 3.0 cm × 3.2 cm × 3.2 cm field of view. The spatial resolution of the system in the reconstructed image is about 1.0 mm in all directions. The sensitivity of a point source in air is 13.3 counts/s/μCi.
Anesthetized animals were placed inside the FASTSPECT aperture using a translation stage. The 99mTc-labeled agent (0.15 ml, 5–10 mCi/185-370 MBq) was injected via the jugular vein catheter followed by a 0.08 ml saline flush. Beginning immediately upon injection, dynamic images were acquired every 1 min for the first 10 min, followed by acquisitions every 5 min for the next 20 min. Using a look-up-table scheme, a total of twenty-four projections, one from each camera, were generated. In the look-up-table scheme, each scintillation event within the camera’s NaI crystal was registered as the digitized outputs from the camera’s four photomultiplier tubes. In order to estimate energy and interaction position, the four outputs were then compared to the 20-bit look-up table. This table was precalculated using a calibration procedure that involved moving a collimated source across the camera face.
2.6. Image processing
The maximum-likelihood expectation-maximization (ML-EM) reconstruction algorithm was applied to generate three-dimensional images. All images were reconstructed using 100 iterations. The projection model built into this algorithm was generated using a calibration scheme that involved moving an uncollimated source through the imaging system’s field of view and recording the system response at each calibration point. Using SlicerDicer software (PIXOTEC LLC, Renton, WA), three-dimensional images were computed to provide images in a 33×49×49 voxels format, and generate tomographic transaxial, coronal and sagittal slices with one-pixel thickness (1.0 mm). A lower threshold value was set around 70 in a color range from 0 to 255 to display the uptake of radiotracer in the tumor.
In all of the images from 1 to 30 minutes after injection, regions of interest (ROI) over the tumors were created from one coronal slice with the highest accumulation of radioactivity. A ROI was also created over a non-tumor area to determine the radioactivity of the background. The tumor ROI was applied to the dynamic images, which were corrected for activity in the background ROI, for determining time-activity curves. The percent washout rate and radioactive retention at the end of the imaging session relative to the peak uptake of the tumors were calculated.
2.7. Biodistribution measurements
The mice were sacrificed by barbiturate overdose at the end of the imaging session, and samples of tumor, blood, skeletal muscle, heart, lung, liver and kidney were harvested. The tissue samples were weighed. Using a CRC-4 Radioisotope Dose Calibrator (Capintec, Ramsey, NJ), the radioactivity of the samples was measured to calculate the percent injected dose per gram of tissue (%ID/gm). The samples were counted in a gamma well counter adjusted to the 99mTc window when the radioactivity of the sample was lower than 2.0 μCi. Standards prepared from an aliquot of the administered 99mTc dose were counted at the same time to establish a conversion factor to translate the counts per minute from the gamma well counter to μCi.
2.8. Western blot analysis
The tumor samples were excised and frozen in liquid nitrogen. After three days when 99mTc decayed to background level, the samples were transferred and maintained at −80°C. MCF7/S and MCF7/D40 tumor tissues were homogenized in lysis buffer. A BioRad DC (BioRad, Hercules, CA) protein assay was used to determine protein concentration (100mg protein loaded per lane on a 10% SDS-PAGE gel). Completed gel was then transferred to a PVDF membrane and probed with a primary antibody against Pgp, a 1:200 dilution of mouse monoclonal antibody C219 (Signet, Dedham, MA). A rabbit anti-mouse-HRP secondary antibody was used (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and detected with ECL Western blot analysis system (Amersham, Piscataway, NJ).
2.9. Data analysis
All results were expressed as mean ± s.e.m. Comparisons between groups were performed with one-way analysis of variance. Comparisons between two variables within a group were made by means of paired t test. Probability values less than 0.05 were considered significant.
Individual radiotracer clearance curves from the peak uptake were fit using nonlinear regression procedures available in TableCurve 2D® software (Systat Software Inc., Richmond, CA).
2.10. Ethics
All experiments were performed in accordance with the guidelines for animal research from the National Institutes of Health (NIH publication 85-23, revised 1985) and were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Arizona.
3. Results
3.1. Detection of Pgp
Expression of human Pgp in the MCF7/D40 cells was demonstrated in Western blots of tumor cell membrane preparations with C219 antibody against Pgp by the presence of a large band (Fig. 1). No immunodetectable MDR1 Pgp was presented in MCF7/S cells.
Fig. 1.

Expression of Pgp in MCF7/S and MCF7/D40 human breast tumors from 3 representative mice in each group (99mTc-sestamibi, 99mTc-tetrofosmin and 99mTc-furifosmin), as determined by Western blots of plasma membrane preparations with mAb C219. The arrow identifies Pgp at 170kDa.
3.2. Tumor visualization with FASTSPECT imaging
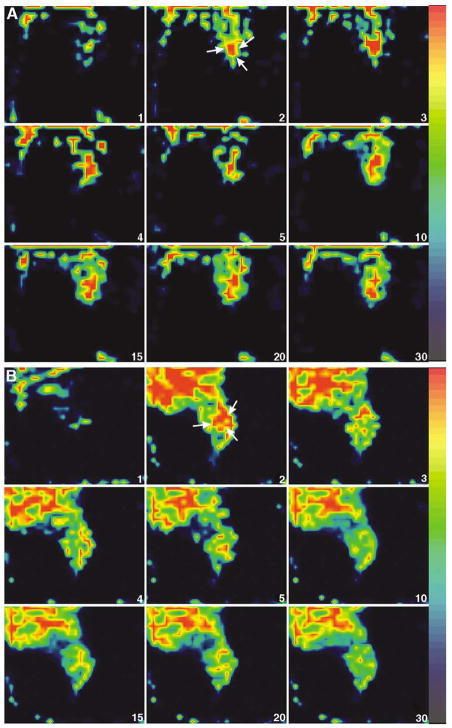
Three-dimensional representation of a reconstructed FASTSPECT data set is shown in Fig. 2, in which a subcutaneous MCF7/S tumor on the right thigh of a mouse was visible clearly on 3-D image and tomographic slice. MCF7/S tumors in the animals were well visualized initially in FASTSPECT images 1–5 minutes after intravenous administration with the three 99mTc-labeled monocationic agents. Dynamic images demonstrated that the radioactive accumulation in the tumors remained for at least 30 minutes post-injection (Figs. 3A, 4A, 5A). In contrast, MCF7/D40 tumors were visualized only 1–5 minutes post-injection of each agent. Then the radioactivity in the tumors quickly dropped to the background level (Figs. 3B, 4B, 5B).
Fig. 2.
Three-dimensional representation (A) of a reconstructed FASTSPECT data set of the right thigh with subcutaneous MCF7/S tumor (arrow) in a mouse (B) 10-min after injection with 99mTc-tetrofosmin. C & D are two selected coronal slices. The tumor (arrow) was visualized on slice D.
Fig. 3.
Representative FASTSPECT dynamic images (selected coronal slices) from two mice with MCF7/S breast tumor (A) and MCF7/D40 tumor (B) using 99mTc-sestamibi. The time after injection is shown in the lower right corner of each image. The MCF7/S tumor (arrow) was visualized about 2 min post-injection and stayed well-defined for at least 30 min. The MCF7/D40 tumor (arrow) was visible for only about 2–3 min post-injection. The tumor weight was 0.084 g (MCF7/S) and 0.15 g (MCF7/D40). The ratio of tumor/muscle was 1.45 (MCF7/S) and 0.72 (MCF7/D40) determined by biodistribution analysis at the end of imaging session.
Fig. 4.
Dynamic images from two mice with MCF7/S breast tumor (A) and MCF7/D40 tumor (B) using 99mTc-tetrofosmin (transaxial slices). The MCF7/S tumor (arrow) was visible clearly from 2 min to 30 min consistently. The MCF7/D40 tumor (arrow) was well identified at 2 min post-injection. After 3 min, the radioactivity in the tumor quickly dropped to the background level. The tumor weight was 0.09 g (MCF7/S) and 0.04 g (MCF7/D40). The ratio of tumor/muscle was 1.31 (MCF7/S) and 0.59 (MCF7/D40), respectively.
Fig. 5.
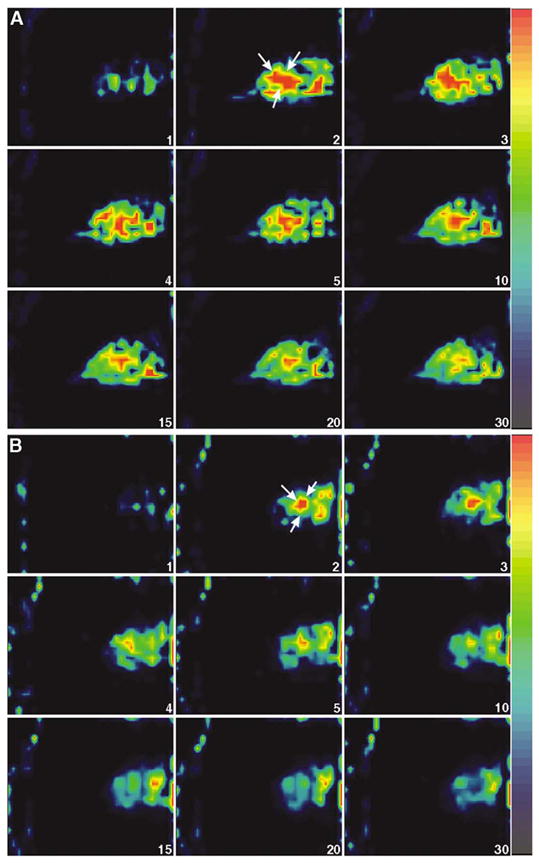
99mTc-furifosmin dynamic images (sagittal slices) from two mice with MCF7/S breast tumor (A) and MCF7/D40 tumor (B). The MCF7/S tumor (arrow) became visible 2–5 min and remained detectable until 30 min post-injection. The MCF7/D40 tumor (arrow) was visualized 2–5 min post-injection. The tumor weight was 0.11 g (MCF7/S) and 0.07 g (MCF7/D40). The ratio of tumor/muscle was 0.93 (MCF7/S) and 0.41 (MCF7/D40), respectively.
3.3. Kinetics of monocationic agents in MCF7 tumors
Fig. 6 shows washout curves of 99mTc-labeled monocationic agents in MCF7/S and MCF7/D40 human breast-tumor xenografts. When tumor activity was normalized as a percentage of peak activity during clearance, the three agents exhibited biphasic clearance curves in MCF7/S and MCF7/D40 tumors. The early phase showed fast clearance and the late phase showed slow clearance. By fitting each individual curve using the TableCurve calculation, biexponential equations were found to best fit the clearance curves for each group. The best fit equation was: y=a*exp(−bx)+c*exp(−dx). There were no significant differences in the half-time values (t1/2, time to reach half of initial activity, minutes) calculated by TableCurve fitting for the early phase among the three imaging agents in MCF7/S and MCF7/D40 (Table 1). The late-phase t1/2 values for the three agents in MCF7/D40 were significantly shorter than the values in MCF7/S. In the MCF7/D40 tumors, 99mTc-furifosmin and 99mTc-tetrofosmin demonstrated a significantly shorter t1/2 in the late phase compared to 99mTc-sestamibi, but there was no difference between 99mTc-furifosmin and 99mTc-tetrofosmin.
Fig. 6.
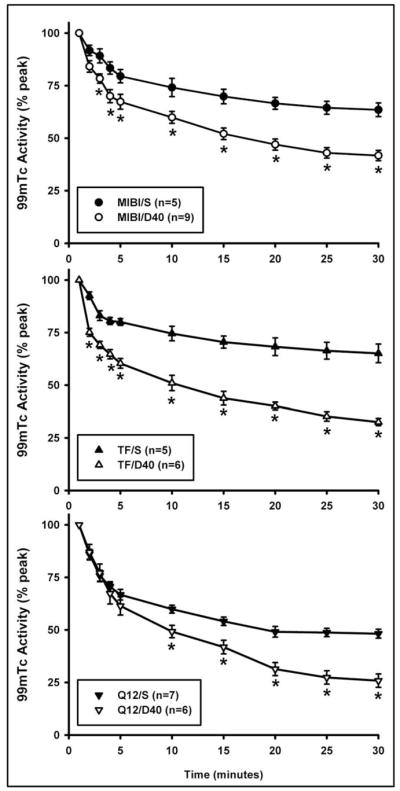
99mTc-sestamibi (MIBI), 99mTc-tetrofosmin (TF) and 99mTc-furifosmin (Q12) clearance curves from MCF7/S and MCF7/D40 breast tumors. The radioactivity is plotted as percent of the peak activity in the tumor. S = MCF7/S; D40 = MCF7/D40.
Table 1.
Biexponential tumor clearance half-time (min)
| Sestamibi MCF7/S |
MCF7/D40 | Tetrofosmin MFCF7/S |
MCF7/D40 | Furifosmin MCF7/S |
MCF7/D40 | |
|---|---|---|---|---|---|---|
| Early Phase | 3.7 ± 0.7 | 2.6 ± 0.6 | 2.1 ± 0.7 | 1.4 ± 0.3 | 2.7 ± 0.4 | 2.4 ± 0.4 |
| Late Phase | 205.6 ± 50.6 | 84.3 ± 12.1* | 194.5 ± 40.5 | 42.7 ± 4.0*† | 106.6 ± 12.5†‡ | 37.5 ± 8.8*† |
man ± s.e.m.,
= p < 0.05 compared to MCF7/S,
p < 0.05 compared to MIBI,
p < 0.05 compared to TF.
The clearance of the three agents from the MCF7/D40 tumors was faster than that from the MCF7/S tumors. The 30-min fractional washout rates (% peak activity) at the end of the imaging sessions are shown in Fig. 7. The washout rates in MCF7/D40 were significantly greater than those in MCF7/S (MIBI: 58.3±2.4 vs. 36.5±3.3; TF: 67.5±1.7 vs. 34.9±4.4; Q12: 74.2±3.2 vs. 51.8±2.1, p<0.05). 99mTc-furifosmin exhibited a greater washout rate than 99mTc-tetrofosmin in only the MCF7/S tumors, but it had a greater washout rate than 99mTc-sestamibi in both the MCF7/S and MCF7/D40 tumors. The washout rate of 99mTc-tetrofosmin was greater than that of 99mTc-sestamibi in MCF7/D40 tumors only. As a result of faster washout, the radioactive retention (% peak) 30 minutes after injection in the MCF7/D40 tumors was significantly lower than that in the MCF7/S tumors (MIBI: 41.7±2.4 vs. 63.5±3.3; TF: 32.5±1.7 vs. 65.1±4.4; Q12: 25.8±3.2 vs. 48.2±2.1) (p<0.05, respectively). 99mTc-furifosmin demonstrated significantly less retention than 99mTc-sestamibi and 99mTc-tetrofosmin in the MCF7/S tumors after 30 minutes of washout. There was no significant difference between 99mTc-sestamibi and 99mTc-tetrofosmin retention in the MCF7/S tumors.
Fig. 7.
99mTc-sestamibi, 99mTc-tetrofosmin and 99mTc-furifosmin fractional washout (%) from MCF7/S and MCF7/D40 tumors. * p<0.05 compared to 99mTc-sestamibi; + p<0.05 compared to 99mTc-tetrofosmin.
3.4. Biodistribution data
The average tumor-weight for the 38 mice was 0.11±0.01 gram. No difference was found in tumor weight between the MCF7/S and MCF7/D40 tumors imaged with the three agents (MIBI: 0.09±0.04 vs.0.14±0.03; TF: 0.09±0.03 vs. 0.14±0.03; Q12: 0.12±0.04 vs. 0.08±0.01, p>0.05, respectively). The biodistribution data are shown in Table 2. In comparison to the MCF7/D40 tumors, the three agents exhibited significantly higher radioactive accumulation (%ID/gm) in the MCF7/S tumors (p<0.05). 99mTc-tetrofosmin showed lower radioactive accumulation compared to 99mTc-sestamibi and 99mTc-furifosmin in MCF7/D40 tumors. Blood activity of 99mTc-furifosmin was significantly higher than that of 99mTc-sestamibi and 99mTc-tetrofosmin (p<0.05). As a result, the tumor-to-blood (T/B) ratios were significantly lower for 99mTc-furifosmin (p<0.05). There was no difference between the tumor-to-blood ratios of 99mTc-sestamibi and 99mTc-teterofosmin. In contrast to T/B ratios, the tumor-to-muscle (T/M) ratios did not differ among the three agents (p>0.05 for all comparisons). Both the T/B and T/M ratios in the MCF7/D40 tumors were significantly lower than in the MCF7/S tumors. The radioactivity of 99mTc-tetrofosmin in the liver was significantly lower than either 99mTc-sestamibi or 99mTc-furifosmin (p<0.05).
Table 2.
Biodistribution data (%ID/gm)
| Sestamibi MCF7/S |
MCF7/D40 | Tetrofosmin MCF7/S |
MCF7/D40 | Furifosmin MCF7/S |
MCF7/D40 | |
|---|---|---|---|---|---|---|
| Tumor | 1.64 ± 0.26* | 0.73 ± 0.09* | 1.34 ± 0.26 | 0.53 ± 0.03* | 1.69 ± 0.36 | 0.79 ± 0.06* |
| Blood | 0.14 ± 0.02 | 0.19 ± 0.05 | 0.12 ± 0.01 | 0.13 ± 0.01 | 0.69 ± 0.05 | 0.66 ± 0.05 |
| Muscle | 0.99 ± 0.12 | 1.10 ± 0.09 | 1.09 ± 0.14 | 0.99 ± 0.08 | 1.58 ± 0.13 | 1.61 ± 0.32 |
| Lung | 1.32 ± 0.26 | 1.96 ± 0.19 | 2.28 ± 0.51 | 1.56 ± 0.16 | 2.62 ± 0.16 | 2.52 ± 0.33 |
| Heart | 15.43 ± 0.36 | 13.80 ± 1.59 | 20.22 ± 0.91 | 16.91 ± 2.27 | 17.18 ± 0.86 | 17.93 ± 2.81 |
| Liver | 21.38 ± 1.48 | 16.41 ± 1.26 | 8.91 ± 0.95 | 7.90 ± 1.83 | 12.72 ± 0.99 | 15.02 ± 2.32 |
| Kidneys | 37.93 ± 1.95 | 40.28 ± 4.35 | 34.22 ± 1.32 | 29.53 ± 2.57 | 34.36 ± 2.43 | 37.58 ± 4.70 |
| Tumor/Blood | 12.32 ± 2.44 | 5.36 ± 0.72* | 11.52 ± 2.18 | 4.10 ± 0.23* | 2.58 ± 0.61 | 1.21 ± 0.07* |
| Tumor/Muscle | 1.66 ± 0.21 | 0.67 ± 0.05* | 1.20 ± 0.14 | 0.55 ± 0.04* | 1.12 ± 0.26 | 0.55 ± 0.08* |
mean ± s.e.m.,
p < 0.05 compared to MCF7/S.
4. Discussion
In this study, in vivo properties of 99mTc-sestamibi, 99mTc-tetrofosmin and 99mTc-furifosmin were assessed and compared in the same SCID mouse model bearing human breast tumors with or without MDR expression identified by Western blotting analysis. The imaging results showed that washout rates for all three monocationic agents were significantly greater in drug-resistant MCF7/D40 tumors than in drug-sensitive MCF7/S tumors. 99mTc-furifosmin exhibited greater washout than 99mTc-sestamibi from both MCF7/S and MCF7/D40 tumors. 99mTc-tetrofosmin’s washout was greater than 99mTc-sestamibi in MCF7/D40 tumors only.
99mTc-sestamibi was the first radiotracer used for studying the expression of Pgp. In this and previous studies using in vitro or in vivo tumor models [11,13,28], faster clearance of 99mTc-sestamibi was observed in MCF7 breast tumors that expressed MDR1 Pgp, as compared with malignancies that did not express MDR. Scintigraphic data in patients with breast cancer demonstrated that 99mTc-sestamibi washout rates from breast cancers overexpressing MDR1 Pgp were 2.7-fold faster than those from cancers not expressing elevated levels of the transporter [15]. Tumor uptake was significantly lower in lesions with Pgp expression and showed an inverse correlation with Pgp expression in patients with untreated breast cancer [15,29,30].
The mechanisms of uptake and retention of 99mTc-sesta-mibi and other cationic analogs have been studied extensively in a variety of models in vitro. Passive influx of this lipophilic cation is driven by the transmembrane potentials generated in living cells. It is sequestered reversibly within mitochondria by the serial thermodynamic driving forces of the negative plasma membrane and mitochondrial inner membrane potentials. Net cell content of the cationic agent is a function of passive potential-dependent influx and transporter-mediated efflux. 99mTc-sestamibi interacts with a common transport domain on Pgp shared by other substrates. The decreased level of the substrates is the result of enhanced efflux due to over-expression of the membrane transports. Hypotonic extracellular ATP, Cl− and K+ gradients in rodent multidrug-resistant cells do not modulate Pgp-mediated 99mTc-sestamibi transport. However, alterations in lipid bilayer structure inhibit the efflux transport function of Pgp and allow the cationic agent to bypass Pgp as it passes through the bilayer [31].
99mTc-tetrofosmin has been tested in several tumor models for recognition as a transport substrate by Pgp. 99mTc-tetrofosmin is a cationic compound containing a peripheral alkoxy with potential advantages as an avid transport substrate for Pgp detection. In doxorubicin-resistant human MCF7 breast tumor cell lines, tetrofosmin uptake was much less than in wild-type drug-sensitive cells [13,32]. In the drug-resistant cell lines, the in vitro accumulation of 99mTc-tetrofosmin was qualitatively similar to that observed with 99mTc-sestamibi, although the absolute levels were much lower. In other words, 99mTc-tetrofosmin and 99mTc-sestamibi did not differ significantly in their behavior. Quantitative data from patients with infiltrating ductal breast cancer demonstrated that tetrofosmin is capable of detecting Pgp function in vivo as effectively as sestamibi [12]. In malignant glioma cells, 99mTc-tetrofosmin demonstrated higher uptake and greater efflux than 99mTc-sestamibi [33]. It was reported that effective inhibition following administration of highly potent and specific MDR1 modulators could be detected by 99mTc-tetrofosmin [13,32,34]. In the current in vivo study with xenografted breast tumors, the radioactive washout and retention of 99mTc-sestamibi and 99mTc-tetrofosmin did not differ significantly in the MCF7/S drug-sensitive tumors. In MCF7/D40 drug resistant tumors, however, 99mTc-tetrofosmin exhibited not only significantly greater washout than in the MCF7/S tumors, but also greater washout than 99mTc-sestamibi. The difference that we observed in the washout of the two agents from the drug-resistant tumors might be related to differences in biological behavior of these two 99mTc-labeled complexes. Though both 99mTc-tetrofosmin and 99mTc-sestamibi possess significant membrane potential-dependent uptake, only a fraction of 99mTc-tetrofosmin accumulates inside the mitochondria and most localizes in the cytoplasm [35,36]. In contrast, most 99mTc-sestamibi accumulation occurs inside the mitochondria because of the large negative potential [37–39]. 99mTc-tetrofosmin does not respond in as robust a manner as 99mTc-sestamibi, which is a high-fidelity probe of trans-membrane potential [18,34].
99mTc-tetrofosmin offers possible clinical advantages over other 99mTc-labeled cationic compounds, such as room temperature reconstitution from a lyophilized kit, rapid clearance from the blood and liver, and a greater degree of renal elimination [40,41]. In our SCID mouse model, the liver radioactivity of 99mTc-tetrofosmin was about half that of 99mTc-sestamibi 30 minutes after injection. Thus, 99mTc-tetrofosmin may not only be a good breast tumor marker similar to 99mTc-sestamibi, but also a sensitive agent to detect MDR1 Pgp expression as an avid transport substrate.
99mTc-Q complexes, which contain multiple alkoxy substitutions on the Schiff base or phosphine ligands or both, have been tested as avid transport substrates recognized by the human MDR1 Pgp [42]. Furifosmin (Q12), a member of the class of 99mTc-Q complexes, demonstrated suitable functional imaging of multidrug resistance in breast tumors in vitro and in vivo in tumor-bearing rats [10]. In rats bearing breast tumors with MDR expression, tumor radioactivity was significantly lower than that in the rats without MDR expression. In MCF7 cell lines, the accumulation of 99mTc-furifosmin in the doxorubicin-resistant cells was lower compared to the sensitive cells, but there was no consistent response to Pgp modulation as in 99mTc-sestamibi and 99mTc-tetrofosmin [13]. In the current study with SCID mice bearing breast tumors, the washout of 99mTc-furifosmin was significantly greater from MCF7/D40 than from MCF7/S, which is similar to results obtained with 99mTc-furifosmin in the tumor-xenografted rats [10]. The greater washout of 99mTc-furifosmin from the drug-resistant tumors seen on images was confirmed by the post-mortem bio-distribution data, in which the radioactive accumulation in the MCF7/D40 tumors was 2.1-fold lower than in the MCF7/S tumors. 99mTc-furifosmin showed much greater washout than 99mTc-sestamibi in the MCF7/D40 tumors. However, the washout of 99mTc-furifosmin was also significantly greater than that of 99mTc-sestamibi in the MCF7/S tumors. 99mTc-furifosmin was cleared more slowly from the blood than 99mTc-sestamibi and 99mTc-tetrofosmin, resulting in higher blood radioactivity and lower tumor-to-background ratios. For functional imaging of MDR in human cancer, an ideal agent should have low nonspecific binding, high distinction in net uptake between drug-sensitive cells and drug-resistant tumor cells, and high enhancement of uptake in resistant cells after treatment with a MDR modulator [42]. Thus, based on the available results in the literature and the current study, 99mTc-furifosmin may meet the MDR-targeting requirements, but its clinical imaging properties might not be as desirable as those of 99mTc-sestamibi or 99mTc-tetroformin.
A biexponential function was found in the present study to provide the best fit for describing the efflux of 99mTc-labeled monocationic agents, rather than the monoexponential function described by Ballinger and Muzzammil [10,11]. A rapid early clearance phase followed by a slow second phase was observed. In the early phase, which primarily reflects perfusion and blood clearance, the half-time of the three agents did not differ between the drug sensitive (MCF7/S) or resistant (MCF7/D40) tumors. In the second phase, which primarily reflects cellular efflux, the half-time of each agent in MCF7/D40 was significantly shorter than in MCF7/S. The principal differences among these agents were in late-phase washout. The second-phase clearance of furifosmin and tetrofosmin was faster than that of 99mTc-sestamibi in MCF7/D40 tumors; 99mTc-furifosmin and 99mTc-tetrofosmin has similar second-phase clearance. This result suggests that 99mTc-tetrofosmin is comparable to 99mTc-sestamibi in localizing human breast tumor, and more desirable than 99mTc-sestamibi in recognizing Pgp expression.
One of the important aspects of the present study is the ability to quantitatively recognize multidrug resistance using high-resolution tomographic imaging of mice with small tumor xenografts. Planar gamma-camera imaging can detect large tumor xenografts in mice using agents such as 99mTc-sestamibi. However, a large amount of cell necrosis in the oversized xenografted tumor may exist, accompanied by inefficient blood supply and hypoxia. This may significantly change the kinetics of radiopharmaceuticals in the tumors. Thus, small tumor xenografts would be preferable for recognition of MDR using noninvasive gamma-ray imaging. Standard planar gamma-camera imaging provides limited spatial resolution of objects less than 1 cm, so its utility for imaging small tumor xenografts is reduced. Pinhole collimation improves gamma camera’s spatial resolution and makes it possible to perform tomographic imaging in small animals. The feasibility of functional imaging using a pinhole SPECT system has been demonstrated in the mouse brain [43]. Conventional single-pinhole imaging usually suffers a poor sensitivity. In order to better meet the sensitivity and resolution requirements for effective small animal imaging, multiple-pinhole collimation has been adopted in specially designed or modified SPECT systems. The high-resolution SPECT system used in the present study, FASTSPECT, is a dedicated 4-D imaging system with stationary camera modules and a stationary multiple-pinhole aperture [24–26]. For tomographic imaging, no uniformity and center-of-rotation corrections are required and the artifact of inaccurate center-of-rotation is not present. There is no dead-space between detectors when the object (e.g. tumor) is localized within the effective 3.0 cm × 3.2 cm × 3.2 cm field of view.
The advantages of FASTSPECT include fast, repeated tomographic imaging ability, high sensitivity, and high spatial resolution. Thus, a rapid sequence of 3-dimensional images can be effectively and repeatedly obtained for imaging recognition of multidrug resistance in mice with small tumor xenografts. In the study reported by Muzzammil et al [11], the tumor weight was about 0.45 grams after 2–3 months growth, which corresponded to a tumor diameter of 9.5–11.5 mm. In the current study, the average volume of MCF7 xenografted breast tumors was 0.11 grams after 10–14 days growth. Dynamic images of such small tumors, with a relatively short growing period, are not practical with a planar gamma camera or conventional rotating SPECT system.
5. Conclusions
The results in this study demonstrate the feasibility of using the three 99mTc-labeled monocationic agents with FASTSPECT for rapidly characterizing Pgp expression in human breast tumor xenografts in vivo. Tetrofosmin behaves similarly to sestamibi in terms of uptake and retention kinetics in drug-sensitive human breast tumors, but has greater washout in tumors with Pgp expression. Thus, tetrofosmin may meet or exceed the MDR-targeting properties of sestamibi, theoretically making it suitable for clinical MDR recognition. The results support the ongoing investigation of the three monocationics as agents for noninvasively optimizing MDR modulators in breast tumor models. The novel imaging system, FASTSPECT, provides a solution-specific approach with high spatial resolution and fast dynamic acquisition for tumor imaging in small animals. The new technology makes it possible to image events in small tumors as early as 10 days after inoculation in SCID mice. FASTSPECT played a major role in this study in functional identification of MDR in mice with xenografted tumors.
Acknowledgments
The authors wish to acknowledge Bethany Skovan, Gillian Paine and Henry Allan Toppin for assistance in establishing the animal model, and Brenda K Baggett for her expertise in preparing tissues for Western Blotting. We thank Amersham Health and Mallinckrodt Medical Inc. for kindly providing us with tetrofosmin (Myoview™) and furifosmin. This work was supported by NIH grants P41RR14304 and R24CA83148.
References
- 1.Pastan I, Gottesman M. Multiple-drug resistance in human cancer. N Engl J Med. 1987;316:1388–93. doi: 10.1056/NEJM198705283162207. [DOI] [PubMed] [Google Scholar]
- 2.Woodhouse JR, Ferry DR. The genetic basis of resistance to cancer chemotherapy. Ann Med. 1995;27:157–67. doi: 10.3109/07853899509031953. [DOI] [PubMed] [Google Scholar]
- 3.Gottesman MM, Pastan I. The multidrug transporter, a double-edged sword. J Biol Chem. 1988;263:12163–6. [PubMed] [Google Scholar]
- 4.Bellamy WT. P-glycoproteins and multidrug resistance. Annu Rev Pharmacol Toxicol. 1996;36:161–83. doi: 10.1146/annurev.pa.36.040196.001113. [DOI] [PubMed] [Google Scholar]
- 5.Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 6.Germann UA, Pastan I, Gottesman MM. P-glycoproteins: mediators of multidrug resistance. Semin Cell Biol. 1993;4:63–76. doi: 10.1006/scel.1993.1008. [DOI] [PubMed] [Google Scholar]
- 7.Ling V. P-glycoprotein: its role in drug resistance. Am J Med. 1995;99:31–4. doi: 10.1016/s0002-9343(99)80283-6. [DOI] [PubMed] [Google Scholar]
- 8.Higgins CV, Callaghan T, Linton KJ, Rosenberg MF, Ford RC. Structure of the multidrug resistance P-glycoprotein. Semin Cancer Biol. 1997;8:135–42. doi: 10.1006/scbi.1997.0067. [DOI] [PubMed] [Google Scholar]
- 9.Piwnica-Worms D, Chiu ML, Budding M, Kronauge JF, Kramer RA, Croop JM. Functional imaging of multidrug-resistant P-glycoprotein with an organotechnetium complex. Cancer Res. 1993;53:977–84. [PubMed] [Google Scholar]
- 10.Ballinger JR, Muzzammil T, Moore MJ. Technetium-99m-furifosmin as an agent for functional imaging of multidrug resistance in tumors. J Nucl Med. 1997;38:1915–9. [PubMed] [Google Scholar]
- 11.Muzzammil T, Ballinger JR, Moore MJ. 99mTc-sestamibi imaging of inhibition of the multidrug resistance transporter in a mouse xenograft model of human breast cancer. Nucl Med Commun. 1999;20:115–22. doi: 10.1097/00006231-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Sun SS, Hsieh JF, Tsai SC, Ho YJ, Kao CH. Technetium-99m tetrofosmin mammoscintigraphy findings related to the expression of P-glycoprotein mediated multidrug resistance. Anticancer Res. 2000;20:1467–70. [PubMed] [Google Scholar]
- 13.Muzzammil T, Moore MJ, Ballinger JR. In vitro comparison of sestamibi, tetrofosmin, and furifosmin as agents for functional imaging of multidrug resistance in tumors. Cancer Biother Radiopharm. 2000;15:339–46. doi: 10.1089/cbr.2000.15.339. [DOI] [PubMed] [Google Scholar]
- 14.Del Vecchio S, Ciarmiello A, Salvatore M. Scintigraphic detection of multidrug resistance in cancer. Cancer Biother Radiopharm. 2000;15:327–37. doi: 10.1089/cbr.2000.15.327. [DOI] [PubMed] [Google Scholar]
- 15.Del Vecchio S, Ciarmiello A, Potena MI, Carriero MV, Mainolfi C, Botti G, Thomas R, Cerra M, D’Aiuto G, Tsuruo T, Salvatore M. In vivo detection of multidrug-resistant (MDR1) phenotype by technetium-99m sestamibi scan in untreated breast cancer patients. Eur J Nucl Med. 1997;24:150–9. doi: 10.1007/BF02439547. [DOI] [PubMed] [Google Scholar]
- 16.Del Vecchio S, Ciarmiello A, Salvatore M. Clinical imaging of multidrug resistance in cancer. Q J Nucl Med. 1999;43:125–31. [PubMed] [Google Scholar]
- 17.McGoron AJ, Gerson MC, Biniakiewicz DS, Roszel NJ, Washburn LC, Millard RW. Extraction and retention of technetium-99m Q12, technetium-99m sestamibi, and thallium-201 in isolated rat heart during coronary academia. Eur J Nucl Med. 1997;24:1479–86. doi: 10.1007/s002590050177. [DOI] [PubMed] [Google Scholar]
- 18.Bernard BF, Krenning EP, Breeman WA, Ensing G, Benjamins H, Bakker WJ, Visser TJ, de Jong M. 99mTc-MIBI, 99mTc-tetrofosmin and 99mTc-Q12 in vitro and in vivo. Nucl Med Biol. 1998;25:233–40. doi: 10.1016/s0969-8051(97)00201-1. [DOI] [PubMed] [Google Scholar]
- 19.Tunggal JK, Ballinger JR, Tannock LF. Influence of cell concentration in limiting the therapeutic benefit of P-glycoprotein reversal agents. Int J Cancer. 1998;81:741–7. doi: 10.1002/(sici)1097-0215(19990531)81:5<741::aid-ijc13>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 20.Chen CC, Meadows B, Regis J, Kalafsky G, Fojo T, Carrasquillo JA, Bates SE. Detection of in vivo P-glycoprotein inhibition by PSC 833 using Tc-99m sestamibi. Clin Cancer Res. 1997;3:545–52. [PubMed] [Google Scholar]
- 21.Bakker M, van der Graaf WT, Piers DA, Franssen EJ, Groen HJ, Smit EF, Kool W, Hollema H, Muller EA, De Vries EG. 99mTc-Sestamibi scanning with SDZ PSC 833 as a functional detection method for resistance modulation in patients with solid tumours. Anticancer Res. 1999;19:2349–53. [PubMed] [Google Scholar]
- 22.Ballinger JR, Hua HA, Berry BW, Firby P, Boxen I. 99mTc-sestamibi as an agent for imaging P-glycoprotein-mediated multi-drug resistance: in vitro and in vivo studies in a rat breast tumour cell line and its doxorubicin-resistant variant. Nucl Med Commun. 1995;16:253–7. doi: 10.1097/00006231-199504000-00156. [DOI] [PubMed] [Google Scholar]
- 23.Muzzammil T, Moore MJ, Hedley D, Ballinger JR. Comparison of (99m)Tc-sestamibi and doxorubicin to monitor inhibition of P-glycoprotein function. Br J Cancer. 2001;84:367–73. doi: 10.1054/bjoc.2000.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein WP, Barrett HH, Pang IW, Patton DD, Rogulski RR, Sain JD, Smith W. FASTSPECT: Electrical and mechanical design of a high-resolution dynamic SPECT imager, Conference Record of the 1995 IEEE. Nucl Sci Symp Medd Img. 1995;2:931–3. [Google Scholar]
- 25.Kastis GK, Barber HB, Barrett HH, Gifford HC, Pang IW, Patton DD, Sain JD, Stevenson GD, Wilson GW. High resolution SPECT imager for three-dimensional imaging of small animals (abstract) J Nucl Med. 1998;39(suppl):9P. [Google Scholar]
- 26.Liu Z, Kastis GA, Stevenson GD, Barrett HH, Furenlid LR, Kupinski MA, Patton DD, Wilson DW. Quantitative analysis of acute myocardial infarct in rat hearts with ischemia-reperfusion using a high-resolution stationary SPECT system. J Nucl Med. 2002;43:933–9. [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor CW, Dalton WS, Parrish PR, Gleason MC, Bellamy WT, Thompson FH, Roe DJ, Trent JM. Different mechanisms of decreased drug accumulation in doxorubicin and mitoxantrone resistant variants of the MCF7 human breast cancer cell line. Br J Cancer. 1991;63:923–9. doi: 10.1038/bjc.1991.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molteni SN, Seregni E, Botti C, Martinetti A, Ferrari L, Crippa F, Bombardieri E. The breast cancer cell line MCF7 as a model of 99mTc-SestaMIBI, 99mTc-tetrofosmin and 99mTc-Medronate incorporation. Anticancer Res. 1999;19:255–9. [PubMed] [Google Scholar]
- 29.Del Vecchio S, Ciarmiello A, Pace L, Potena MI, Carriero MV, Mainolfi C, Thomas R, D’Aiuto G, Tsuruo T, Salvatore M. Fractional retention of technetium-99m sestamibi as an index of P-glycoprotein expression in untreated breast cancer patients. J Nucl Med. 1997;38:1348–51. [PubMed] [Google Scholar]
- 30.Takamura Y, Miyoshi Y, Taguchi T, Noguchi S. Prediction of chemotherapeutic response by Technetium 99m–MIBI scintigraphy in breast carcinoma patients. Cancer. 2001;92:232–9. doi: 10.1002/1097-0142(20010715)92:2<232::aid-cncr1314>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 31.Piwnica-Worms D, Rao VV, Kronauge JF, Croop JM. Characterization of multidrug resistance P-glycoprotein transport function with an organotechnetium cation. Biochemistry. 1995;34:12210–20. doi: 10.1021/bi00038a015. [DOI] [PubMed] [Google Scholar]
- 32.Ballinger JR, Bannerman J, Boxen I, Firby P, Hartman NG, Moore MJ. Technetium-99m-tetrofosmin as a substrate for P-glycoprotein: in vitro studies in multidrug-resistant breast tumor cells. J Nucl Med. 1996;37:1578–82. [PubMed] [Google Scholar]
- 33.Perek N, Prevot N, Koumanov F, Frere D, Sabido O, Beauchesne P, Dubois F. Involvement of the glutathione S-conjugate compounds and the MRP protein in Tc-99m-tetrofosmin and Tc-99m-sestamibi uptake in glioma cell lines. Nucl Med Biol. 2000;27:299–307. doi: 10.1016/s0969-8051(00)00085-8. [DOI] [PubMed] [Google Scholar]
- 34.Chen WS, Luker KE, Dahlheimer JL, Pica CM, Luker GD, Piwnica-Worms D. Effects of MDR1 and MDR3 P-glycoproteins, MRP1, and BCRP/MXR/ABCP on the transport of (99m)Tc-tetrofosmin. Biochem Pharmacol. 2000;60:413–26. doi: 10.1016/s0006-2952(00)00341-5. [DOI] [PubMed] [Google Scholar]
- 35.Arbab AS, Koizumi K, Toyama K, Araki T. Uptake of technetium-99m-tetrofosmin, technetium-99m-MIBI and thallium-201 in tumor cell lines. J Nucl Med. 1996;37:1551–6. [PubMed] [Google Scholar]
- 36.Platts EA, North TL, Pickett RD, Kelly JD. Mechanism of uptake of technetium-tetrofosmin. I: Uptake into isolated adult rat ventricular myocytes and subcellular localization. J Nucl Cardiol. 1995;2:317–26. doi: 10.1016/s1071-3581(05)80076-5. [DOI] [PubMed] [Google Scholar]
- 37.Piwnica-Worms D, Kronauge JF, Chiu MI. Uptake and retention of hexakis (2-methoxyisobutyl isonitrile) technetium (I) in cultured chick myocardial cells. Mitochondrial and plasma membrane potential dependence. Circulation. 1990;82:1826–38. doi: 10.1161/01.cir.82.5.1826. [DOI] [PubMed] [Google Scholar]
- 38.Chiu ML, Kronauge JF, Piwnica-Worms D. Effect of mitochondrial and plasma membrane potentials on accumulation of hexakis (2-methoxyisobutylisonitrile) technetium(I) in cultured mouse fibroblasts. J Nucl Med. 1990;31:1646–53. [PubMed] [Google Scholar]
- 39.Carvalho PA, Chiu ML, Kronauge JF, Kawamura M, Jones AG, Holman BL, Piwnica-Worms D. Subcellular distribution and analysis of technetium-99m-MIBI in isolated perfused rat hearts. J Nucl Med. 1992;33:1516–22. [PubMed] [Google Scholar]
- 40.Kelly JD, Forster AM, Higley B, Archer CM, Booker FS, Canning LR, Chiu KW, Edwards B, Gill HK, McPartlin M. Technetium-99m-tetrofosmin as a new radiopharmaceutical for myocardial perfusion imaging. J Nucl Med. 1993;34:222–7. [PubMed] [Google Scholar]
- 41.Munch G, Neverve J, Matsunari I, Schroter G, Schwaiger M. Myocardial technetium-99m-tetrofosmin and technetium-99m-sestamibi kinetics in normal subjects and patients with coronary artery disease. J Nucl Med. 1997;38:428–32. [PubMed] [Google Scholar]
- 42.Crankshaw CL, Marmion M, Luker GD, Rao V, Dahlheimer J, Burleigh BD, Webb E, Deutsch KF, Piwnica-Worms D. Novel technetium (III)-Q complexes for functional imaging of multidrug resistance (MDR1) P-glycoprotein. J Nucl Med. 1998;39:77–86. [PubMed] [Google Scholar]
- 43.Acton PD, Choi SR, Plössl K, Kung HF. Quantification of dopamine transporters in the mouse brain using ultra-high resolution single-photon emission tomography. Eur J Nucl Med Mol Imaging. 2002;29:691–8. doi: 10.1007/s00259-002-0776-7. [DOI] [PubMed] [Google Scholar]