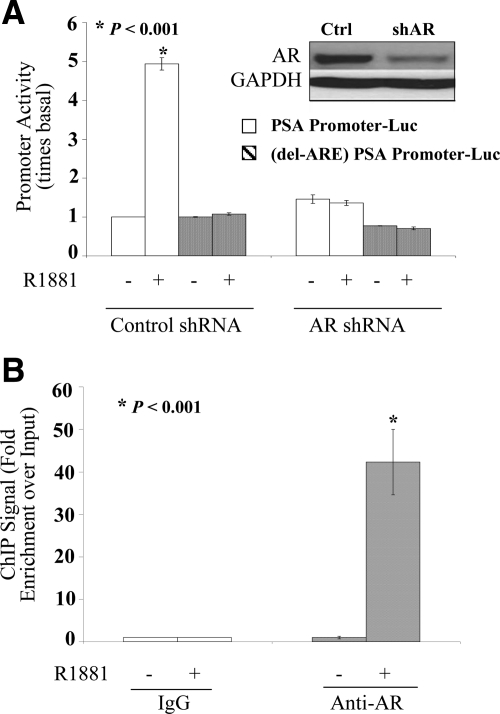
Fig. 3.
Androgen dependence for functional and physical association of AR with classical response elements in LP50 cells. A, Hormone-depleted LP50 cells were transfected by nucleofection with either the PSA promoter (6.1-kb fragment)-Luc reporter construct or with the same construct in which the multiple dispersed AREs were removed by deletion of only the AREs that include −4366 to −3874 nt and an additional internal deletion of −170 to −159 nt (delARE-PSA promoter-Luc) as indicated. The cells were cotransfected with AR shRNA or nontargeting shRNA (negative control). In addition, all wells were cotransfected with the Renilla luciferase control plasmid. R1881 (1 nm) or vehicle was added to the culture media 12 h after transfection; 72 h after transfection, the samples were harvested either to measure luciferase activity or for Western blot analysis. The promoter activity values are plotted as the ratio to the basal activity of the corresponding promoter in the nontargeting shRNA controls. Triplicate samples were included in each experimental set. A, inset, Western blotting showing the expression of AR or GAPDH (loading control). B, Hormone-depleted LP50 cells were treated with R1881 (1 nm) or vehicle for 2 h and subjected to ChIP assay using either an AR-specific rabbit antibody or negative control rabbit IgG. The immunoprecipitated DNA fragments as well as input DNA were amplified. Enrichment for fragments covering the major ARE enhancer region of PSA (−4366 to −3874 nt) within the immunoprecipited DNA compared with input DNA was measured by real-time PCR targeting the enhancer region. Specificity of the immunoprecipitation was also confirmed by the lack of enrichment of an irrelevant target sequence within the open reading frame of the PSA gene.