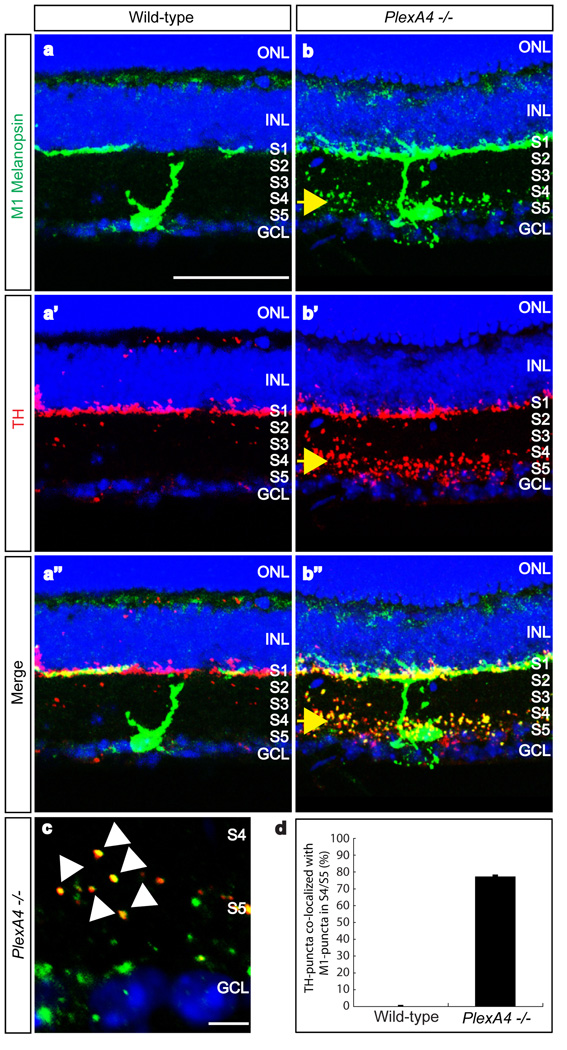
Figure 2. PlexinA4 controls dendritic targeting of M1-type ipRGCs within the IPL, but not co-localization of dopaminergic amacrine cell and ipRGC processes.
a–a”, b–b”, Double-immunostaining using antibodies directed against the C-terminus of rat melanopsin (a and b, green) and against TH (a’ and b’, red) of wild-type (a–a”) and PlexA4−/− (b–b”) adult retina sections (merged in a” and b”). Ectopic dendritic processes of M1-type ipRGCs were observed in the S4/S5 sublaminae of PlexA4−/− retinas, as were aberrant dopaminergic amacrine processes (yellow arrows, b–b”, n=4 mutant animals). Wild-type M1-type ipRGC dendritic processes and dopaminergic amacrine cell processes are only observed in S1 (a–a”).
c, High-magnification of S4/S5 in PlexA4−/− retinas double-immunostained with anti-C-terminal melanopsin and anti-TH. Most TH-positive puncta are co-localized with melanopsin-positive puncta (white arrowheads).
d, Quantification of ectopic TH-positive puncta co-localized with the ectopic M1-type melanopsin puncta in S4/S5 of PlexA4−/− retinas. Nearly 76% (194 TH-positive puncta among a total of 254 puncta) of the ectopic TH-positive puncta were co-localized with ectopic M1-type melanopsin puncta in S4/S5 (76.4±1.2% co-localization). In wild-type retinas, almost no TH-positive puncta were observed in S4/S5. Error bar indicates SEM (n=3 animals per genotype). Scale bars: 50 µm in a for a–a” and b–b”, and 5 µm in c.