Fig. 4.
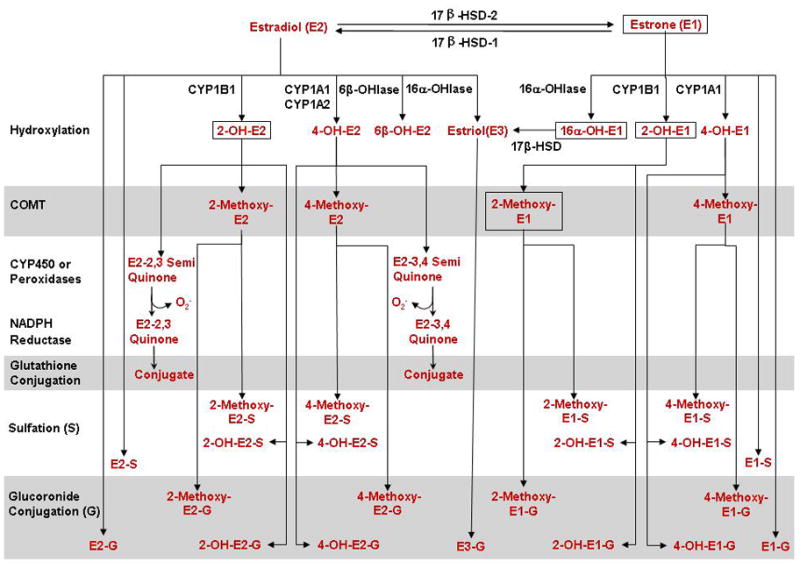
Estrogen metabolism. E2 may undergo conjugation by glucoronidation or sulfation for excretion. E2 may also be hydroxylated in ring A (2-OH, 4-OH), ring B (6β-OH), and ring D (16α-OH) and then conjugated for excretion. Also, the 2-OH and 4-OH metabolites may be methylated by COMT, forming 2- and 4-methoxy-E2. Alternatively, 2-OH and 4-OH metabolites may be subject to peroxidases then reductases forming E2-quinones, with simultaneous generation of superoxides leading to oxidative stress. E2 is transformed into E1 by 17β-HSD-2, and reversibly from E1 to E2 by 17β-HSD-1. E1, in turn, may be sulfated or gluocoronide conjugated for excretion. E1 may also be hydroxylated in ring D (16α-OH) and ring A (2-OH, 4-OH). 16α-OH-E1 may be converted to E3 by 17β-HSD. 2-OH-E1 and 4-OH-E1 may be subject to methylation by COMT, forming 2- and 4-methoxy-E1 which are then sulfated for excretion. 2-OH-E1 and 4-OH-E1 can also be sulfated or gluocoronide conjugated for excretion. The compounds enclosed in boxes are the major estrogen metabolites excreted in urine.
