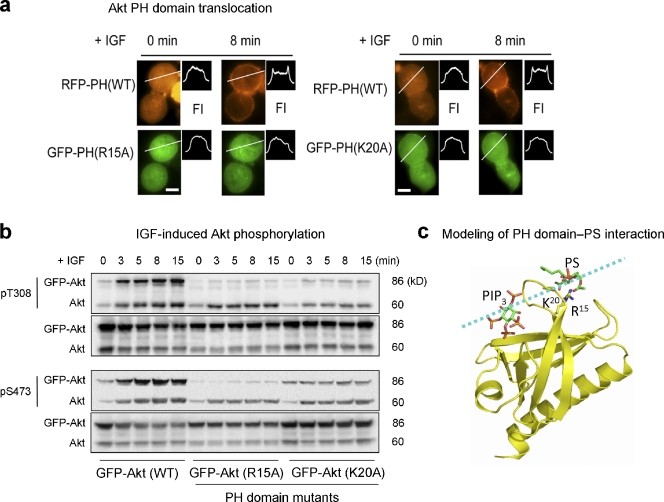
Figure 5.
Akt membrane translocation and phosphorylation impaired by disrupting Akt–PS interaction via mutation of the PS-binding residues in the PH domain. (a) Representative micrographs showing IGF-induced membrane translocation of Neuro 2A cells coexpressing RFP-PH (WT) and GFP-PH (R15A or K20A). The membrane translocation is depicted by a representative fluorescence intensity profile across a transverse section in indicated cells (FI). Bars, 10 µm. (b) Time course of IGF-induced Akt phosphorylation at T308 and S473 in Neuro 2A cells expressing GFP-Akt WT or GFP-Akt mutants. (c) Computer modeling depicting binding of PS to R15 and K20 located outside the PIP3-binding pocket in the PH domain. The broken line indicates a putative membrane surface. The lowest energy conformations generated by using a Lamarckian genetic algorithm (Morris et al., 1998) showed the putative binding of PS to R15 and K20 via hydrogen bonding.