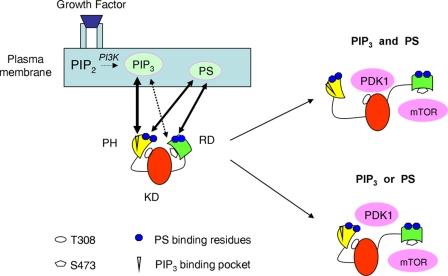
Figure 9.
Schematic presentation of the PS involvement in Akt activation. PS and PIP3 jointly regulate the membrane binding and interdomain conformational changes of Akt for unfolding the PH and RD to expose T308 and S473 for phosphorylation and activation. When the growth factor receptor is stimulated, PIP3 is generated in the membrane, which in turn triggers translocation of cytosolic Akt to the plasma membrane through the specific binding of PIP3 to the PH domain. Although necessary, the PIP3–PH interaction alone is not sufficient for securing Akt binding to the plasma membrane. Interaction of PS with the PS-binding residues in the PH domain outside the PIP3-binding pocket is also required for the membrane binding and conformational changes to expose T308 for phosphorylation by PDK1. Near the plasma membrane, presumably after or concurrent with PIP3–PH binding, the PS-binding residues in the RD also interact with PS, resulting in an open conformation, allowing S473 phosphorylation by mTORC2. Although direct binding of PIP3 with the RD is minimal, PIP3 can also induce an open RD conformation to expose S473 for phosphorylation. The involvement of KD has not been evaluated separately.