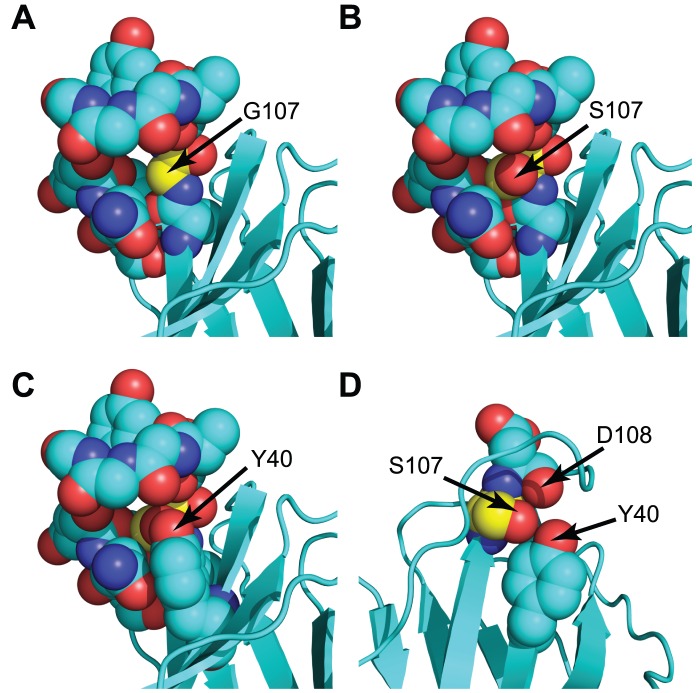
Figure 3. Modeling of a G107S mutation in the 172.10 TCR.
(A) Arrow and yellow colored carbon atoms indicate the position of G107 within 172.10. A gap is noticeable within the center of the CDR3β. (B) A G107S substitution, modeled without altering the atomic positions of other amino acids, demonstrates the potential for the S hydroxymethyl to fill the gap. (C) Addition of the Y40 side chain to the structure in (B) using coordinates from the original 172.10 structure further shows accommodation of the G107S substitution. (D) Removal of side chains of select amino acids and rotation of the structure relative to (C) demonstrates the juxtaposition and potential for H bonding between the inserted S107 hydroxyl, the D108 carboxyl O, and the Y40 side chain hydroxyphenyl in a manner corresponding to that observed in the AHIII 12.2 and other TRBV13-2− TCR crystal structures (see Fig. 2b).