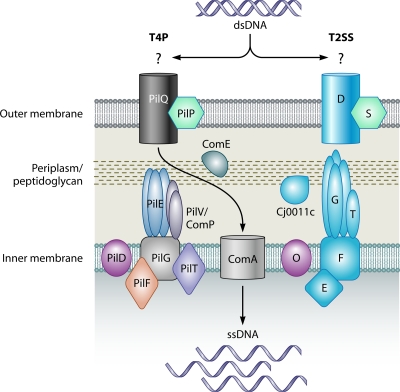
FIG. 3.
Components of the T4P and T2SS involved in transformation. (Left) Model of the T4P system, based on those previously reported (90, 91). Indicated components are named after the N. gonorrhoeae T4P. The outer membrane-spanning pore complex consists of PilQ and is assisted by the PilP pilot protein. The pilus/pseudopilus structure is composed of major (PilE) and minor (PilV or ComP) pilin proteins, which are processed by the prepilin peptidase, PilD. The T4P adhesin, PilC, associates with the distal end of the pilus structure (not shown). Pilus/pseudopilus biogenesis is also facilitated by the traffic NTPase PilF and the inner membrane protein PilG. Another traffic NTPase, PilT, is involved in disassembly of the pilus/pseudopilus. During twitching motility, the pilus complex polymerizes to facilitate attachment to a nearby surface. Once attached, the pilus complex depolymerizes to result in bacterial locomotion. In a similar manner, donor DNA may be taken into the cell by binding to an unidentified DNA receptor followed by retraction of the competence pseudopilus structure. Once inside the periplasmic space, the donor DNA may then be processed and translocated into the cytoplasm through ComA. DNA translocation may involve the periplasmic protein ComE. (Right) The T2SS contains several components that are homologous to the T4P system. The components shown are referred to by their respective letter designations, and the model is based on those reported previously (91, 516). Note that not all known T2SS components are shown and that some components may be species specific. Assisted by the S pilot protein, protein D forms the outer membrane-spanning pore complex. The pseudopilus-like structure is formed by G, as well as other minor pseudopilins, collectively represented by T, which are processed by the prepilin peptidase O. Other components involved in pseudopilus assembly are the inner membrane protein F and the traffic NTPase, E. The secretion-activating signal may be transmitted from E to the outer membrane by proteins C, M, and L (not shown). Known components of the C. jejuni Cts T2SS-like transformation system are depicted in blue. These components include homologs of the pore protein D (CtsD), the major and minor pseudopilins G (CtsG) and T (CtsT), the inner membrane protein F (CtsF), and the traffic NTPase E (CtsE) (641). Additionally, the periplasmic protein Cj0011c may be involved in transport of DNA in the periplasm. It is proposed that the T2SS pseudopilus acts as a piston, forcing secreted effectors from the periplasm into the extracellular environment. Although the mechanism for T2SS-mediated DNA uptake is currently unknown, it is possible that once donor DNA is bound, the T2SS works in reverse to transport DNA across the outer membrane and into the recipient cell.