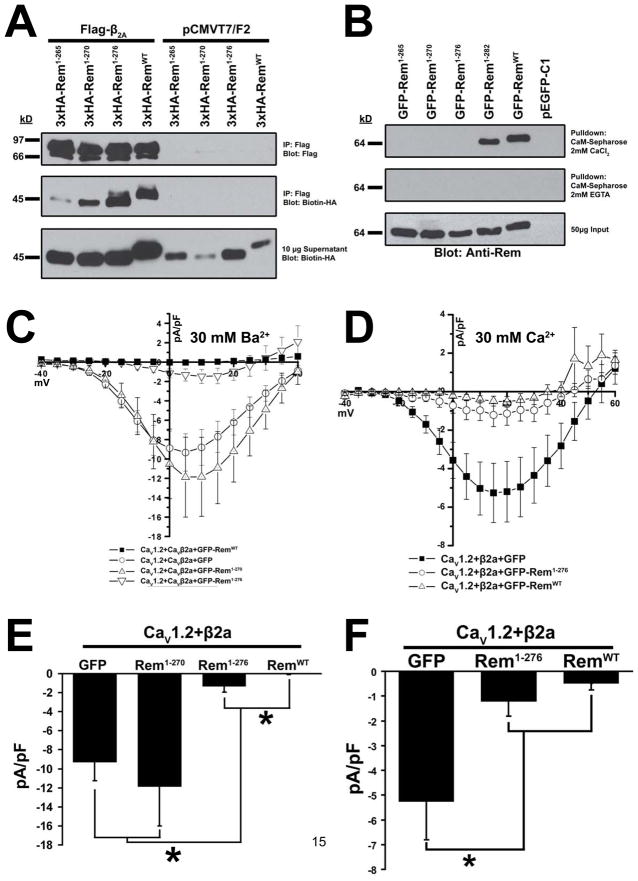
Figure 3. β2a association is not sufficient for Rem-mediated Ca2+ channel regulation.
(A) HEK293 cells were transfected with 3xHA-Rem truncations and either empty pCMVT7F2 (FLAG) vector or Flag-CaVβ2a. Co-immunoprecipitation was performed with Flag antibody and interaction with Rem examined by immunoblotting with biotinylated anti-HA antibody. (B) TsA201 cells were transfected with plasmids expressing GFP-Rem1-265, GFP-Rem1-270, GFP-Rem1-276, GFP-Rem1-282, GFP-RemWT, or empty pEGFP-C1 as control. Lysates were pulled down onto calmodulin-sepharose beads in the presence of 2 mM CaCl2 or 2.5 mM EGTA, beads were boiled to release bound protein, and the ability of Rem truncations to associate with calmodulin was examined by immunoblotting with anti-Rem antibody. (C) HEK293 cells were transfected with plasmids expressing CaV1.2, Flag-CaVβ2a, and either GFP-Rem1-270, GFP-Rem1-276, GFP-RemWT or empty pEGFP-C1 as control. Current through CaV1.2+CaVβ2a complex was examined using the whole-cell patch clamp configuration in the presence of 30 mM Ba2+. (D) TsA201 cells were transfected with plasmids expressing CaV1.2, Flag-CaVβ2a, and either GFP-Rem1-276, GFP-RemWT or empty pEGFP-C1 as control. Current through CaV1.2+CaVβ2a complex was examined using the whole-cell patch clamp configuration in the presence of 30 mM Ca2+. (E) Currents at 5 mV from Figure 3C. A significant difference (p<0.05) between treatments is denoted by asterisks. (F) Currents at 5 mV from Figure 3D. A significant difference (p<0.05) between treatments is denoted by asterisks.