Figure 5.
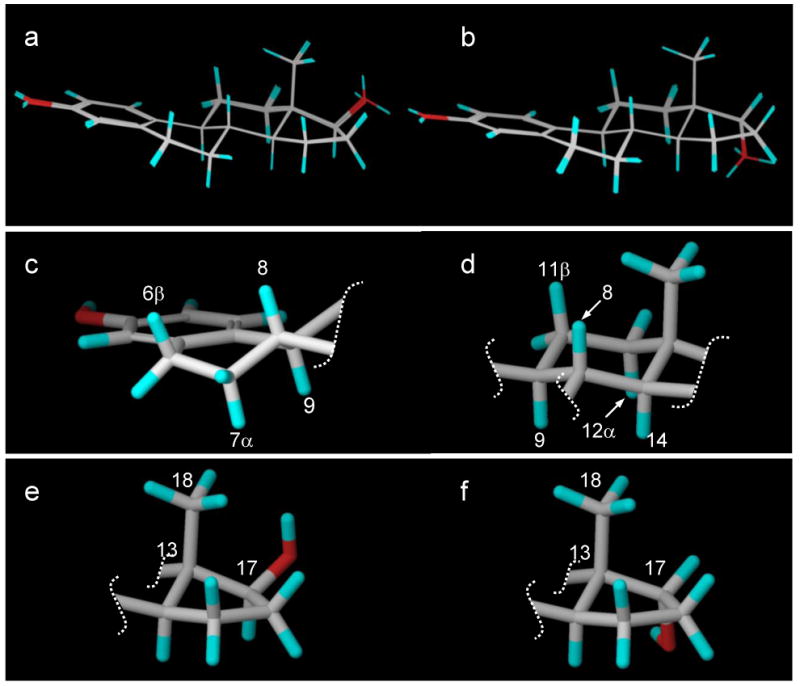
(a) The 20 low-energy conformers of 17β-estradiol (E2). (b) The 20 low-energy conformers of 17α-estradiol. (c) The unsaturated B-ring half-chair showing trans coplanar diaxial orientations for H6β with H7α and for H8 with H9. (d) The C-ring chair with trans coplanar diaxial orientations for H11β with H9 and H12α as well as for H8 with H9 and H14. (e) The D-ring for 17β-estradiol (E2). (f) The D-ring for 17α-estradiol.
