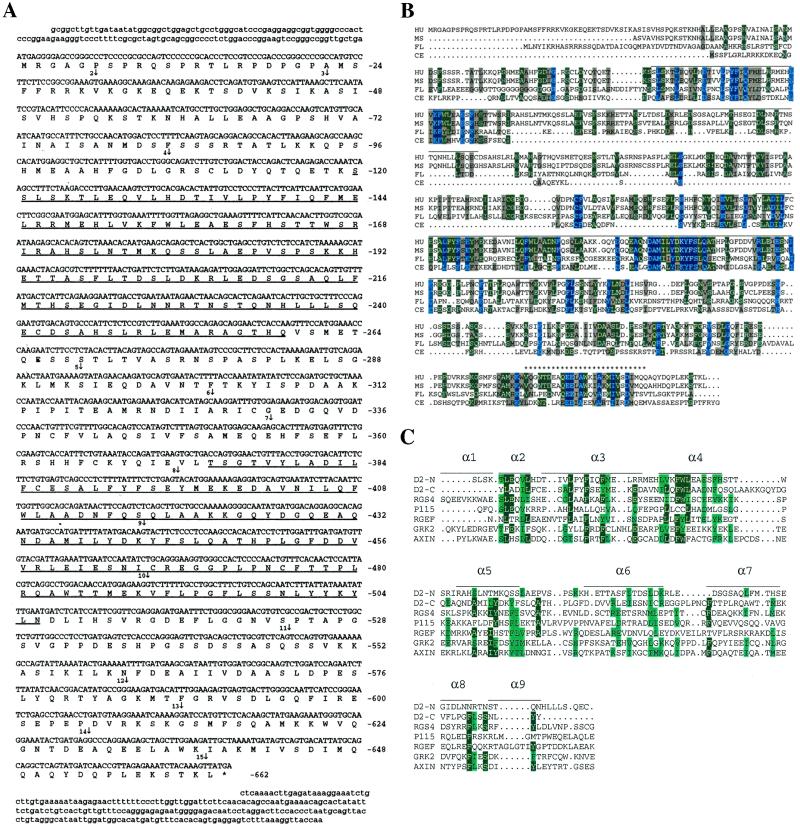
Figure 1.
(A) cDNA and deduced protein sequence of human D-AKAP2. Amino acid numbers are shown on the right. The two putative RGS domains are underlined. The star indicates a stop codon. Vertical arrows indicate the beginning of each numbered exon. Exons 1 and 15 extend beyond the cDNA sequence we have. (B) Comparison of the sequence of D-AKAP2 from four species: HU, human; MS, mouse; FL, fly, drosophila; CE, C. elegans. The PKA-binding region is indicated by stars (*). Horizontal lines above the sequence indicate the two putative RGS domains. (C) Alignment of the RGS domains of the N- and C-terminal portions of D-AKAP2. Comparison of the RGS domains of various RGS molecules in a multiple sequence alignment were generated by CLUSTAL W. The sequences include the N-terminal domain of D-AKAP2 (amino acids 125–242), C-terminal domain of D-AKAP2 (amino acids 379–502), rat RGS4 (amino acids 62–174, GenBank no. AF117211), human axin (amino acids 88–207, GenBank no. AF009674), P115 (amino acids 48–166, GenBank no. U64105), Drosophila Rho GEF2 (amino acids 930-1052, GenBank no. AF032870), and human GRK2 (amino acids 54–171, GenBank no. P25098). Also illustrated is the secondary structure of the largely α-helical RGS4, based on the x-ray crystal structure described by Tesmer et al. (24). α-Helical domains are represented by horizontal lines.