Abstract
BACKGROUND:
The efficacy of Mycophenolate mofetil (MMF) plus interferon beta-1a (IFNB-1a) in treatment of relapsing-remitting multiple sclerosis (RRMS) was evaluated.
METHODS:
This was a pilot study with randomized, double-blinded, placebo-controlled design. Patients with RRMS and Expanded Disability Status Scale score (EDSS) of < 6.0 were included. Those with relapse within the previous two months and prior use of immunomodulatory/immunosuppressive drugs were excluded. Patients were randomized into MMF (n = 13) and placebo (n = 13) groups and received weekly intramuscular IFNB-1a plus either MMF or placebo. MMF started by 500 mg/d for one week and weekly escalated by 500 mg/d, until target divided dose of 2000 mg/d and continued for 12 months. Radiologic and clinical assessments were performed at baseline and then at month 12.
RESULTS:
After one year of therapy, difference between the two groups in number of new T2 lesions was not statistically significant (0.54 ± 0.77 in MMF vs. 1.85 ± 3.2 in placebo group, p = 0.169). Two patients in the placebo group had gadoliniumenhanced lesions and one patient had relapse. There were 3 patients in each group with more than one point progression in EDSS. Common side effect in the MMF group included gastrointestinal upset, but no patient discontinued the treatment.
CONCLUSIONS:
Combination of MMF with IFNB-1a in patients with RRMS is well tolerated, but the efficacy of such combination was not statistically significant in this pilot study and deserves further investigation with a larger sample size and a longer follow-up.
Keywords: Mycophenolate Mofetil, Interferon Beta-1a, Immunosuppressive, Relapsing-Remitting Multiple Sclerosis
Multiple sclerosis (MS) is an immunemediated demyelinating disease of the central nervous system. The pathophysiology of MS is complex; however, the most widely accepted hypothesis is that it begins as an autoimmune disorder mediated by autoreactive lymphocytes.1,2 Based on the accepted role of autoimmunity in the pathogenesis of MS, a variety of immunomodulatory and immunosuppressive drugs have been investigated for the treatment of MS.3,4
A number of different medications have been approved for the treatment of relapsing-remitting multiple sclerosis (RRMS) including three IFNB preparations (Avonex®, Rebif®, Betaseron®), glatiramer acetate, mitoxantrone, and Natalizumab.5,6 Despite promising efficacy of IFNB in the therapy of RRMS, a large number of patients are poor responders to IFNB agents or glatiramer acetate. In these patients, combination therapy may be a promising therapeutic strategy.7–9
Data are available on the effects of Mycophenolate mofetil (MMF), a powerful inhibitor of lymphocyte proliferation, in combination with IFNB in the treatment of RRMS. In an open-labeled study, RRMS patients with a history of breakthrough disease activity after 6 months of IFNB-1a treatment received MMF as adjunctive therapy and the results suggested beneficial effects of combining MMF and IFNB in patients with poor response to IFNB alone.9 Retrospective data also documented the safety profile of MMF alone or in combination with IFNB or glatiramer acetate.10,11 Despite these promising reports, there is a lack of controlled trials in this regard. Also, there is no report on the effect of such combination therapy in new cases of MS. In this placebo-controlled trial, the effects of MMF in combination with IFNB-1a in the treatment of RRMS were evaluated.
Methods
Setting and Patients
This pilot study, with randomized, double-blinded (patient and assessor), placebo-controlled design, was conducted from April 2008 to September 2009 in Isfahan MS Society (IMSS), Isfahan, Iran. Calculated sample size per group was 13, considering α = 0.05, study power = 80%, effect size = 1, and SD = Range/6. Eligible patients were recruited from the registered patients at IMSS. Inclusion criteria were age of 18 to 55 years, clinically definite MS (according to McDonald criteria)12 with disease duration of less than two years, RRMS disease course, and baseline Expanded Disability Status Scale score (EDSS) of less than 6.0. Those with a clinical relapse within two months prior to enrollment, prior use of immunomodulatory or immunosuppressive drugs, women who were pregnant, lactating, or of childbearing age who did not consent to approved contraceptive use during the study, those with abnormal blood tests performed during the screening studies, and patients with other major medical illnesses (e.g. cancer, significant gastrointestinal disease, immunodeficiency, or other autoimmune diseases) were excluded from the study. Informed consent was obtained from all patients after full explanation of the study aim and protocol and the ethical approval was obtained from the Ethics Committee of Isfahan University of Medical Sciences, Isfahan, Iran.
Intervention
Patients were randomized into MMF (n = 13) and placebo (n = 13) groups using table of random numbers generated by random allocation software.13 Weekly intramuscular IFNB-1a started for all patients. Patients in the MMF group received oral MMF (500 mg Tablet) started at 500 mg daily for one week, weekly escalated by 500 mg daily, until a target dose of 2000 mg daily (1000 mg every 12 hours) was achieved and then continued it for 12 months. Placebo was administered with the same daily schedule as of MMF in the placebo arm. Compliance was checked during the study by the treating physician in interviews with patients.
Assessments
The primary outcome of the study was the number of new T2 lesions and new Gd-enhancing lesions in MRI evaluation after one year of therapy. The MRI was performed with a Philips 1.5T scanner. Two radiologists, blinded to the treatment arms, were responsible for MRI evaluations. The secondary outcomes included relapse rate and changes in the EDSS score. Physical examination, monitoring of adverse effects, and laboratory studies including complete blood count with differential, liver function tests (LFT), blood urea nitrogen; and creatinine were performed at baseline and then at months 1, 2, 3, 6, and 12. EDSS assessment and brain MRI were performed at baseline and then after one year of therapy. Relapses during the study were documented by a single neurologist and treated by intravenous methylprednisolone 1g/day for three consecutive days.
Statistical Analysis
Data were analyzed using SPSS version 16.0. (Chicago, IL). Comparisons between the two groups were performed using independent t-test and chisquare test. A p value of less than 0.05 was considered significant.
Results
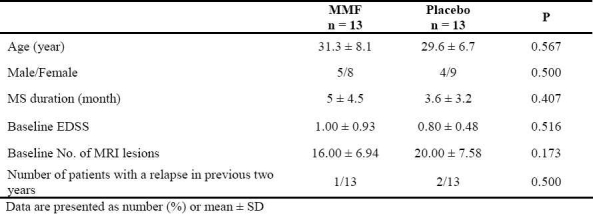
During the study period, 92 patients with RRMS were screened, from which 66 patients were excluded; 60 previously received IFNB, 40 with more than 2 years duration, and 10 patients had received immunosuppressive drugs. Finally, 26 patients were included and randomized into MMF and placebo groups, and all of them completed the study. There were no significant differences between the two groups in baseline characteristics (Table 1).
Table 1.
Baseline characteristics of the MMF and placebo groups
Primary and Secondary Outcomes
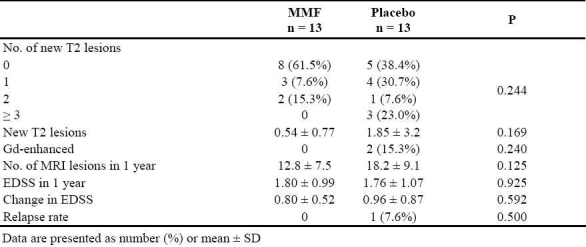
After one year of therapy, the difference between the two groups in the number of new T2 lesions in MRI was not statistically significant (p = 0.169). Two patients in the placebo group had Gd-enhancement and one patient had a relapse while no patient in the MMF group experienced such findings Table 2. Number of patients with more than one point progression in EDSS was 3 in each group and there was no significant difference between the groups in change in EDSS scores (Table 2). No abnormality was observed in CBC results, LFT, or renal function tests during the study in any patient. Observed side effects in MMF group mostly included various degrees of gastrointestinal upset in all and transient diarrhea (3 days) in one patient, but no patient discontinued the treatment.
Table 2.
Disease characteristics in the two groups before and after the therapy
Discussion
The aim of the present study was to evaluate the effects of MMF in combination with IFNB-1a in the treatment of RRMS. According to the results, MMF in combination with IFNB-1a has no more significant effects over IFNB-1a alone on clinical (relapse rate, EDSS) or paraclinical (number of new T2 or Gd-enhancment lesions in MRI) measures in relatively new cases of MS without history of previous IFNB therapy.
Current disease-modifying therapies for RRMS include IFNB preparations and glatiramer acetate, but in considerable amount of patients, these therapies are only partially effective and patients usually have break-through disease activity despite therapy with these agents.6 Therefore, there is a need for additional treatment options in MS, e.g. combination therapies to enhance the effectiveness of these relatively safe drugs.
Evidence has suggested the ability of MMF in a treatment of a variety of inflammatory disorders including systemic lupus erythematosus, myastheniagravis, chronic inflammatory demyelinating polyneuropathy, polymyositis, and neuromyelitis optica.14 Evidence on the efficacy and safety of MMF in the treatment of MS are also available. Open-labeled studies involving MS patients treated with MMF suggested tolerability and potential efficacy of this drug.10,11 In a recently published randomized trial, Frohman et al. compared MMF with IFNB and found similar effects of MMF compared with IFNB on inflammatory brain MRI indices including the cumulative mean number of new Gd-enhancing lesions, new T2 lesions, and combined active lesions and disability measures including EDSS scores and multiple sclerosis functional composite.15 To the best of our knowledge there is no report from placebocontrolled study on the tolerability and potential efficacy of combining MMF with IFNB. Vermersch and colleagues in an openlabeled pilot safety study evaluated the effect of adding MMF to IFNB-1a in 30 patients with RRMS refractory to IFNB-1a and found clinical and radiographic benefits as well as acceptable safety.9 The results of the present study, however, did not show statistically significant effect of combining MMF with IFNB, which is probably due to the small sample size of the study and relatively short duration of observation. Although data are available on the safety of MMF in MS pa-tients, considering the possible severe side effects of MMF, we could not enroll more patients for investigation. Previous studies have included MS patients who were refractory to IFNB therapy, but in our study we included patients with no history of treatment with IFNB because we aimed to find the effect of such combination in new cases of MS to see if we could prevent relapse and further progression of disease to more progressive types. Therefore, since the differences were not significant between the groups, larger sample size is needed to detect smaller differences. According to the results of the present study, at least 64 patients per group are needed for a more reliable analysis.
Conclusions
The results of this study showed that MMF is a safe medication when combined with IFNB, considering clinical and laboratory measures. However, we were unable to show significant clinical or radiological improvements for the MMF over placebo in MS patients under IFNB therapy. It should be considered that this was a pilot study and had limitations and most importantly the sample size was small. Considering the observed safety of the combination, controlled trials with a larger sample size and longer followups are recommended in this regard.
Conflict of Interests
Authors have no conflict of interests.
Authors’ Contributions
The principal idea was from ME, who also participated in designing and conducting the study, as well as editing the manuscript. MK was the manager of the study and participated in designing and conducting the study, assessments, and writing the draft. AC also participated in conducting the study and editing the draft. AK, NT, and AG did the assessments and AHM participated in writing the draft. All authors have read and approved the final manuscript.
Acknowledgments
This study was supported by a grant from Isfahan University of Medical Sciences (Grang No. 388374). We are thankful to the personnel of Isfahan MS Society specially Mahnaz Saeidipoor, Shahrzad Erfanifard, and Zahra Sarraf who helped us in conducting the study. Also, we are thankful to Dr. Ali Gholamrezaei (Isfahan University of Medical Sciences) for editing this paper.
References
- 1.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343(13):938–52. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 2.Gandhi R, Laroni A, Weiner HL. Role of the innate immune system in the pathogenesis of multiple sclerosis. J Neuroimmunol. 2010;221(1-2):7–14. doi: 10.1016/j.jneuroim.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kieseier BC, Wiendl H, Leussink VI, Stüve O. Immunomodulatory treatment strategies in multiple sclerosis. J Neurol. 2008;255(Suppl 6):15–21. doi: 10.1007/s00415-008-6004-z. [DOI] [PubMed] [Google Scholar]
- 4.Gauthier SA, Buckle GJ, Weiner HL. Immunosuppressive therapy for multiple sclerosis. Neurol Clin. 2005;23(1):247–72. doi: 10.1016/j.ncl.2004.09.007. viii-ix. [DOI] [PubMed] [Google Scholar]
- 5.Chofflon M. Recombinant human interferon beta in relapsing-remitting multiple sclerosis: a review of the major clinical trials. Eur J Neurol. 2000;7(4):369–80. doi: 10.1046/j.1468-1331.2000.00057.x. [DOI] [PubMed] [Google Scholar]
- 6.Rice GP, Incorvaia B, Munari L, Ebers G, Polman C, D'Amico R, et al. Interferon in relapsing-remitting multiple sclerosis. Cochrane Database Syst Rev. 2001;4 doi: 10.1002/14651858.CD002002. CD002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gold R. Combination therapies in multiple sclerosis. J Neurol. 2008;255(Suppl 1):51–60. doi: 10.1007/s00415-008-1008-2. [DOI] [PubMed] [Google Scholar]
- 8.Jeffery DR. Use of combination therapy with immunomodulators and immunosuppressants in treating multiple sclerosis. Neurology. 2004;63(12 Suppl 6):S41–6. doi: 10.1212/wnl.63.12_suppl_6.s41. [DOI] [PubMed] [Google Scholar]
- 9.Vermersch P, Waucquier N, Michelin E, Bourteel H, Stojkovic T, Ferriby D, et al. Combination of IFN beta-1a (Avonex) and mycophenolate mofetil (Cellcept) in multiple sclerosis. Eur J Neurol. 2007;14(1):85–9. doi: 10.1111/j.1468-1331.2006.01562.x. [DOI] [PubMed] [Google Scholar]
- 10.Ahrens N, Salama A, Haas J. Mycophenolate-mofetil in the treatment of refractory multiple sclerosis. J Neurol. 2001;248(8):713–4. doi: 10.1007/s004150170122. [DOI] [PubMed] [Google Scholar]
- 11.Frohman EM, Brannon K, Racke MK, Hawker K. Mycophenolate mofetil in multiple sclerosis. Clin Neuropharmacol. 2004;27(2):80–3. doi: 10.1097/00002826-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclero-sis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58(6):840–6. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 13.Saghaei M. Random allocation software for parallel group randomized trials. BMC Med Res Methodol. 2004;4:26. doi: 10.1186/1471-2288-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iaccarino L, Rampudda M, Canova M, Della Libera S, Sarzi-Puttinic P, Doria A. Mycophenolate mofetil: what is its place in the treatment of autoimmune rheumatic diseases? Autoimmun Rev. 2007;6(3):190–5. doi: 10.1016/j.autrev.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Frohman EM, Cutter G, Remington G, Gao H, Rossman H, Weinstock-Guttman B, et al. A randomized, blinded, parallel-group, pilot trial of mycophenolate mofetil (CellCept) compared with interferon beta-1a (Avonex) in patients with relapsing remitting multiple sclerosis. Ther Adv Neurol Disord. 2010;3(1):15–28. doi: 10.1177/1756285609353354. [DOI] [PMC free article] [PubMed] [Google Scholar]


