Abstract
This article provides an overview of how intestinal epithelial cells (IEC) recognize commensals and how they maintain host-bacterial symbiosis. Endocrine, goblet cells, and enterocytes of the intestinal epithelium express a range of pattern recognition receptors (PRR) to sense the presence of microbes. The best characterized are the Toll-like receptors (TLR) and nucleotide oligomerization domain-like receptors (NLR), which play a key role in pathogen recognition and the induction of innate effectors and inflammation. Several adaptations of PRR signaling have evolved in the gut to avoid uncontrolled and potentially destructive inflammatory responses toward the resident microbiota. PRR signaling in IEC serve to maintain the barrier functions of the epithelium, including the production of secretory IgA (sIgA). Additionally, IECs play a cardinal role in setting the immunosuppressive tone of the mucosa to inhibit overreaction against innocuous luminal antigens. This includes regulation of dendritic cells (DC), macrophage and lymphocyte functions by epithelial secreted cytokines. These immune mechanisms depend heavily on IEC recognition of microbes and are consistent with several studies in knockout mice that demonstrate TLR signaling in the epithelium has a profoundly beneficial role in maintaining homeostasis.
Keywords: homeostasis, immunity, intestinal epithelium, Toll-like receptors
A single layer of epithelial cells separates the inimical contents of the mammalian intestine from the underlying tissues of the body. Excluded are an estimated 1 × 1014 commensal bacteria consisting of at least 160 species of >1,000 identified in one of the most densely populated habitats known in biology (1, 2). The small intestine is organized into crypts and villi to increase the surface area for absorption of nutrients whereas, in the colon, there are no villi and the surface of the epithelium is flat. The entire epithelium is renewed approximately every 5 d in humans because of proliferation and differentiation of the pluripotent stem cells residing in the crypts. The cells migrate upwards and differentiate into three different cell lineages: enterocytes, enteroendocrine cells and mucus producing goblet cells (3). In the small intestine, the stem cells migrating to the bottom of the crypt differentiate into Paneth cells, which produce a range of antimicrobial factors to protect the crypt cells from infection by microorganisms (4). The layer of mucosal tissue directly under the intestinal epithelium, known as the lamina propia (LP), contains B cells (especially sIgA-producing plasma cells), T cells, stromal cells, and antigen-presenting cells (APC) such as macrophages and DC. Additionally, the epithelium contains intraepithelial lymphocytes with unclear ontogeny and reactivity that localize to this site independently of antigen-specific activation in the secondary lymphoid organs. Two radial layers of innervated smooth muscle surround the gut wall and LP to propel the luminal contents through the intestine by peristalsis. The gastrointestinal tract is also enveloped by nerves of the enteric nervous system involved in two-way signaling with the central nervous system. Here, microbe interactions with the epithelium and, in particular, the surface sensory enteroendocrine cells in the small intestine can potentially mediate communication between the brain and the intestine.
Although once considered simply a physical barrier, it is becoming increasingly evident that the epithelium is a crucial regulator of intestinal immune homeostasis. In this respect, the interaction of epithelial cells with microbes and components released by microbes including their metabolites, is a key mediator of the cross-talk between the epithelium and other cell types in the mucosa. The interactions between bacteria and epithelium may differ in the small and large intestine because of anatomical differences and the extent to which the secreted mucus layer covers the epithelium. The mouse colonic mucus consists of two layers extending 150 μm above the epithelial surface with a similar protein composition (5). Whereas the inner layer is densely packed and devoid of bacteria, the outer layer is less dense and colonized by bacteria (5). It is not known whether the mucus in the small intestine also consists of two layers and if it would entirely cover the large surface area of the villi in the small intestine. In addition, the density and composition of bacteria differs substantially between the colon and small intestine, suggesting that microbe–epithelial interactions will be different in each location.
Microbe–epithelial interactions do not necessarily involve intact bacteria because diffusible compounds released from live or dead bacterial cells could also interact with the cognate PRR of the host. The well characterized PRR expressed by epithelial cells include members of the TLR family and intracellular NLR, which can trigger expression of a surprising diversity of chemokines, cytokines, and effectors of innate immunity (Table 1). In this review, we discuss what is known about the role of some of these factors in regulating immune functions, including antigen sampling, B cell function, sIgA production, DC function, and innate immunity.
Table 1.
Chemokines and effectors of innate immunity produced by intestinal epithelial cells
| Effector | Function | Source or ref. |
| APRIL | B cell proliferation inducing ligand promoting Ig class switching | Entrez Gene/GeneRIFs |
| TSLP | Activates and stimulates survival of immune cell types including DC and CD4+ T cells | Entrez Gene/GeneRIFs |
| SLPI | Conferring protection of epithelia from attack by endogenous proteolytic enzymes during immune responses | HPRD (6)* |
| TGF-β | Cytokine involved in cell proliferation, differentiation, apoptosis and coagulation; mainly secreted by macrophages | Entrez Gene/GeneRIFs, HPRD |
| ALPI | Detoxifies lipopolysaccharide and prevents bacterial invasion across the gut mucosal barrier | Entrez Gene/GeneRIFs, HPRD |
| IL-1β | An important mediator of the inflammatory response; is involved in diverse cellular activities including cell proliferation, differentiation and apoptosis. | (7), Entrez Gene GeneRIFs, HPRD |
| IL-7 | Important for B and T cell development and cell survival | Entrez Gene, HPRD |
| IL-8 | T cell chemotactic factor; angiogenic factor; mediates inflammatory responses | HPRD (7, 8) |
| IL-15 | Cytokine that regulates T and natural killer cell activation and proliferation. | Entrez Gene/GeneRIFs, HPRD |
| CCL20 | Chemokine; macrophage inflammatory protein 3 alpha; small inducible cytokine | HPRD |
| CD46 | Type I membrane protein that is a regulatory part of the complement system | This publication |
| CD55 | Glycoprotein involved in the regulation of the complement cascade | Entrez Gene/GeneRIFs, HPRD |
| SOCS3 | Suppresses proinflammatory cytokine signaling; inhibitor of JAK2 and STAT3 signaling | (9) |
| ADM | Suppresses proinflammatory cytokines, chemokines and NO production; inhibits T cell proliferation, induces regulatory T (Treg) cells | Entrez Gene, HPRD |
| POMC | Peptide hormone precursor with roles in energy homeostasis and immune modulation and homeostasis. | HPRD |
| defensins | A family of microbicidal and cytotoxic peptides involved in host defense | (4) |
*Human Protein Reference Database, www.hprd.org.
Expression and Compartmentalization of PRR in the Intestinal Epithelium
The detection of pathogens by the host is achieved through the families of PRR that recognize conserved molecular structures known as pathogen-associated molecular patterns (PAMP) and induce production of innate effector molecules. Because these structures are also found on nonpathogenic microorganisms, the term microbe-associated molecular patterns (MAMP) is increasingly used, particularly in the context of host–commensal interactions. These signaling receptors can be divided into three families: TLR, retinoic acid inducible gene I (RIG-I)-like receptors (RLR), and nucleotide oligomerization domain (NOD)-like receptors (NLR). The TLR family is the best characterized, and 13 receptors have been reported in mice and humans. For TLR, it has been shown that ligand sensing and specificity is achieved through the arrangement and sequence variation in the conserved leucine-rich repeat (LRR) domains. TLR2 can form heterodimers with TLR1 or TLR6 to detect different but related ligands (Table 2). TLR are localized in the cell membrane and/or endosomal membrane components to recognize extracellular and endocytosed MAMP (Table 2). The ubiquitously expressed RIG-I-like receptor (RLR) family of RNA helicases are cytoplasmic proteins that recognize viral RNAs and induce innate antiviral responses, including the activation of proinflammatory cytokines and type I interferon (IFN) (10). The third family, containing >20 cytoplasmic NLR in humans and mice, is divided into four major groups based on the nature of the N-terminal activation domains involved in signal transduction. The NLR characterized to date recognize a wide range of bacterial ligands and toxins as well as certain damage-associated molecular patterns (DAMP) of the host cell (11). NLR proteins can signal through different multicomponent signal complexes to activate alternative signaling pathways, including caspase activation, cell death, and NF-κB leading to cytokine, chemokine, and defensin expression. In the intestine, only the functions of NOD1 and NOD2 have been well characterized, and these NLR respond to the synthetic peptidoglycan components meso-diaminopimelic acid (DAP) and muramyl dipeptide (MDP), respectively (Table 2).
Table 2.
The PRRs, subcellular localization, and recognized ligands
| Receptor | Subcellular localization | Ligand | Origin of ligand |
| TLR2 | Cell surface | Lipoteichoic acid | G (+) bacteria |
| Lipoprotein/ lipopeptides | Various pathogens | ||
| Hemoagglutinin protein | Viruses (Measles Virus) | ||
| Glycosyl-phosphatidylinositols | Parasites (Toxoplasma gondii) | ||
| TLR2/1 | Cell surface | Triacyl lipopeptides | Bacteria and mycobacteria |
| TLR2/6 | Cell surface | Diacyl lipopeptides | Mycobacteria |
| Zymosan | Fungi | ||
| TLR3 | Cellular compartment | dsRNA | Viruses |
| TLR4 | Cell surface | Lipopolysaccharide | G (−) bacteria |
| Envelope proteins | Viruses (Respiratory Syncytial Virus) | ||
| Glycosyl-phosphatidylinositols | Parasites (Toxoplasma gondii) | ||
| TLR5 | Cell surface | Flagellin | Bacteria |
| TLR7/8 | Cellular compartment | ssRNA | Viruses |
| TLR9 | Cellular compartment/cell surface | CpG-containing DNA | Bacteria and viruses |
| TLR11 | Cell surface | Uropathogenic bacteria component | Bacteria (uropathogenic Escherichia coli) |
| Profilin | Parasites | ||
| NOD1 | Cell cytoplasm | Meso-diaminopimelic acid | PGN of G (−) and some G (+) bacteria |
| NOD2 | Cell cytoplasm | Muramyl dipeptide | PGN of G (+) and G (−) bacteria |
| RIG-I | Cell cytoplasm | 5′-triphosphate-bearing RNAs | Viruses |
G (+), Gram-positive; G (−), Gram-negative; dsRNA, double-stranded RNA; ssRNA, single-stranded RNA; PGN, peptidoglycan; Meso-DAP, γ-D-glutamyl-meso-diaminopimelic acid; MDP, muramyl dipeptide NOD1, nucleotide oligomerization domain-like receptor 1; NOD2 nucleotide oligomerization domain-like receptor 2.
The NOD1 receptors are constitutively expressed in a broad range of tissue types, including intestinal cells such as the Caco-2 cell line (12–14). In contrast, NOD2 expression was reported to be absent in epithelial cell lines and human tissue samples and confined to Paneth cells in the small intestine (14–16).
Almost all TLR have been shown to be expressed at the mRNA level in the human colon and small intestine, but our knowledge about their spatial distribution in the epithelium, cell lineage specificity, and function in different parts of the intestine is incomplete (17, 18). Immunohistochemical techniques have shown that TLR2 and TLR4 are expressed at low levels by IEC in normal human colon tissues and predominantly in the crypt epithelial cells (19–22). The compartmentalization of TLR4 to the colonic crypts has also been reported in the mouse (23). In contrast, TLR3 seems to be abundantly expressed only in mature enterocytes of the normal human small intestine and colon. TLR5 is expressed predominantly in the colon (20). In the mouse, immunohistochemical analyses showed that TLR2, TLR4, and TLR5 are localized on both the follicle-associated epithelium (FAE) and the epithelium of the small intestinal villi and crypts (24), but TLR4 expression was relatively low. TLR2 and TLR9 expression is found on both apical and basolateral sides of the FAE but only on the apical membrane of small intestinal IECs in the mouse and human. TLR4 signaling is generally thought to occur at the plasma membrane after the binding of LPS to MD2–TLR4 complexes, but a study in a mouse epithelial cell line showed that TLR4 was located in the Golgi apparatus along with LPS (25). Internalization of LPS was necessary for CXCL2 chemokine induction, suggesting that intracellular TLR4 signaling is required for activation of NF-κB in the epithelium (26).
PRR Signaling in the Intestinal Epithelium
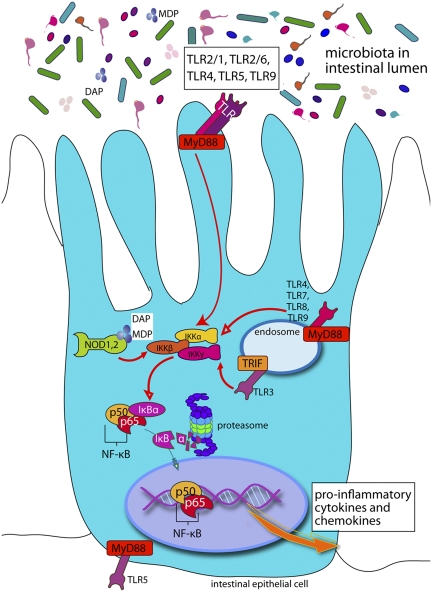
After ligand binding, TLRs recruit adaptor proteins and cellular kinases that trigger downstream signaling cascades, leading to the activation of the MAPK pathway and canonical pathway of NF-κB activation (recently reviewed in refs. 17 and 18). Consequently, NF-κB is translocated into the nucleus, where it induces expression of proinflammatory cytokines and chemokines (Fig. 1). TLR3 and TLR4 can also activate the IFN response factor 3 via recruitment of the adaptor protein TRIF, leading to expression of type 1 interferons. NOD1 and NOD2 signal by acting as a scaffold for the assembly of multicomponent signaling complexes that lead to activation of NF-κB through interactions with receptor interacting protein 2 (RIP2, also known as RICK or CARDIAK) and a serine/threonine kinase (11).
Fig. 1.
Simplified cartoon of PRR signaling in epithelial cells showing only the canonical pathway of NF-κB activation and some of the key receptors, adaptors, and kinases involved. After stimulation of TLRs or NLRs by microbial products, the inhibitor of IkB is phosphorylated, leading to its degradation and the translocation of NF-κB into the nucleus where it activates the expression of cytokines and chemokines and other effectors.
PRR signaling is a crucial aspect of innate defense, but if uncontrolled at mucosal surfaces, it would be pathological. Thus, there must be robust mechanisms in place to avoid chronic stimulation of inflammatory signaling by the resident microbiota while maintaining responsiveness to pathogens. In this respect, the polarization of IEC seems to play a crucial role. Recently, TLR9 activation through the apical and basolateral membranes of IECs was shown to induce distinct transcription responses. The apical activation of TLR9 conferred a homeostatic response and tolerance to subsequent TLR challenges, whereas basal stimulation triggers IκB degradation and activation of the canonical NF-κB pathway (27). In addition, a growing list of inhibitors of TLR signaling in IECs, including IRAK-M, TOLLIP, SIGIRR, A20, and peroxisome proliferator-activated receptor-γ (PPARγ), ensure that chronic inflammatory and potentially destructive TLR responses to MAMP do not occur (28).
Although various mechanisms are in place to dampen PRR signaling in the healthy gut, there is clear evidence from work in TLR knockout mice that a tonic level of signaling is necessary to maintain intestinal homeostasis. For example, mouse knockouts in TLR9, -4, -2, and the adaptor protein MyD88 all showed increased susceptibility to DSS-induced colitis (27, 29). Additionally, germ-free mice and mice treated with multiple antibiotics to reduce the microbial content of the intestine are also more susceptible to DSS-induced colitis but can be protected by administration of agonists for TLR2 and -4 (29).
Epithelial Regulation of Innate Immunity
In addition to promoting digestion and absorption of nutrients, IEC contribute to the mucosal barrier functions by producing a range of antimicrobial factors, including defensins, cathelecidins, calprotectin, and antimicrobial polypeptides such as HIP/PAP (Reg3γ and Reg3β in the mouse) (4, 18). It was not surprising therefore to discover that IEC-specific deletion of TLR4 and NOD1 or MyD88 impaired immunity to bacterial infections (30–32). TLR5 knockout mice have a tendency to develop spontaneous colitis. In these mice, there are more bacteria closely associated with the colon epithelium and increased bacterial translocation to the liver and spleen, indicating a failure to control the microbiota. Additionally, NOD2 polymorphisms in Crohn's disease patients are associated with impaired innate immunity, reduced expression of antimicrobial peptides, and deregulation of immune tolerance to the microbiota (33, 34).
Accumulating evidence indicates that the inducible expression of defensins and other antimicrobial factors depends on TLR or MyD88-dependent signaling (35, 36). Using a mouse transgenic model in which MyD88 was expressed specifically in Paneth cells on a MyD88−/− background, it was shown that Paneth cell production of several antimicrobial factors was MyD88-dependent (35). This model was also used to show that Paneth cell-specific expression of antimicrobial factors is sufficient to limit mucosal uptake of commensal and pathogenic bacteria into the mesenteric lymph nodes (MLN) although it did not impact on the density of luminal bacteria. These findings are consistent with a Paneth cell model where MyD88-dependent TLR signaling triggered by commensal bacteria at the mucosal surface induces expression of several antimicrobial factors that inhibit growth of bacterial pathogens and commensals in the epithelial crypts of the small intestine.
Apart from their antimicrobial activities, the β-defensins are proposed to have additional signaling functions. Human beta defensin-2 (HBD-2), for example, has been shown to be a chemoattractant of immature DC through its interaction with the chemokine receptor-6 (CCR6) (37, 38). Furthermore, HBD-2 may activate epithelial restitution and barrier repair in an autocrine fashion by binding to CCR6 on colonic epithelial cells (39). Murine β-defensin 2 (MBD-2) and human β-defensin 3 have been reported to have effects on DC function by binding to TLR4 and TLR1/2, respectively (37, 40). These studies highlight additional mechanisms by which TLR signaling in epithelial cells can influence both adaptive and innate immunity.
Epithelial Regulation of Antigen Sampling in the Mucosa
The intestinal epithelium is in close proximity to a large number of gut-associated lymphoid cells, most noticeably in the small intestine where aggregates of lymphoid follicles form specialized structures known as Peyer's Patches (PP) (41). Isolated lymphoid follicles (ILFs) are also present throughout the intestinal tract including the colon. The smooth follicular epithelium covering the PP and ILFs contains specialized antigen-sampling cells (called M cells), which take up particulate antigens and specific binding proteins by endocytosis. M cells are devoid of lysosomes, and sampled antigens are transcytosed relatively intact to antigen-presenting cells in the PP lymphoid cells lying in close proximity to the epithelial basal membrane (42). After being primed, naive T and B cells become memory/effector cells and migrate from the efferent lymph vessels of the gut-associated lymphoid tissue (GALT) to the MLN and then via the thoracic duct to peripheral blood to other mucosal effector sites such as the LP. Homing of MLN-primed lymphocytes to distal mucosal sites is controlled by the profile of adhesion molecules and chemokines expressed on the endothelial cells of the gut microvasculature (43). The FAE cells express TLR and express chemokines to attract antigen-presenting subepithelial DC into the dome (apical) area of the PP lymphoid follicles. Injection of bacterial peptidoglycan, lipopolysaccharide, and Pam3Cys (a synthetic agonist of TLR2) results in enhanced transepithelial transport of microparticles by M cells in a dose-dependent manner (24). Furthermore, TLR2 activation induced the matrix metalloproteinase-dependent migration of subepithelial DC into the FAE, but not into villus epithelium. These responses were not observed in TLR2- and -4-deficient mice, demonstrating that the TLR recognition of microbial ligands functions to promote antigen capture and antigen presentation by DC in the PP.
Another mode of antigen uptake by DC located in the LP involves the extension of dendrites (membrane extensions) between epithelial cells to directly sample luminal antigens (25, 26, 44). Remarkably, DC sampling through the epithelium occurs without loss of epithelial integrity because of the creation of tight junction-like complexes between DC and IEC (45). Recent studies showed that active DC sampling is induced by epithelial cell TLR signaling upon exposure to microbial stimuli (46). Epithelial produced chemokines or other factors may play also a role in promoting DC extensions.
Microbe-Epithelial Cell Regulation of Intestinal Secretory IgA
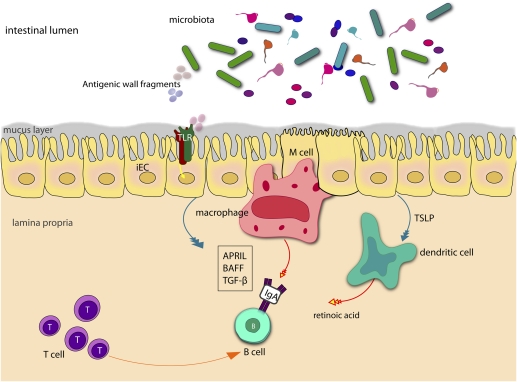
The gut is by far the largest antibody-producing organ in the body, and in humans, >80% of the activated B cells reside in the mucosal tissues (47). In germ-free mice, IgA production is very low but increases considerably after colonization with intestinal bacteria (48, 49). In mucosal tissues, secretory antibody provides a crucial defense against pathogens and also plays a role in shaping the ecology of the microbiota (50, 51). The signals required for class switch recombination of IgM-positive B cells to IgA occur in the PP after which the committed plasma B cells undergo recirculation via the lymph, thoracic duct, and bloodstream to the LP where they secrete IgA. The production of retinoic acid by DC in the PP is one of the key factors driving the class switch recombination of IgM-positive B cells to IgA (52). APRIL, a B cell proliferation-inducing ligand, also stimulates class switch recombination to IgA in mucosal B cells and appears to be necessary for IgA production in vivo. Both APRIL and TGF-β, another important cytokine that signals IgA induction (53–55), are produced by DC, macrophages, activated T cells, and epithelial cells (Fig. 2). Recently, a role for epithelial TLR4 recognition of commensal bacteria in B cell recruitment and IgA class switching was revealed in transgenic mice expressing a constitutively active form of TLR4 in IEC (56). These mice did not have histopathological inflammation but rather striking increases in B cell recruitment and IgA production due to the increased IEC expression of chemokines CCL20, CCL28, and APRIL (56). Another recent study showed that switching to IgA2, a more proteolytically resistant class of IgA, occurs primarily in the colon because of the microbial induction of APRIL and BAFF expression in IEC (Fig. 2) (57). IEC secrete APRIL in response to TLR signaling and also thymic stromal lymphopoietin (TSLP), which stimulates APRIL production by DC (57). Thus, steady-state signaling induced by commensal bacteria appears to contribute to intestinal homeostasis by eliciting production of APRIL and BAFF to promote IgA2 class switching. Once secretory IgA (sIgA) and secretory IgM (sIgM) have been produced in the LP, they are exported to the lumen via the epithelial polymeric immunoglobulin receptor.
Fig. 2.
The role of IEC signaling in B cell class switching. Microbial recognition by IEC leads to the production of the cytokine TGF-β and the TNF-family member APRIL that promote IgA class switching in B cells. DC and macrophages acquire antigens from commensal bacteria promiscuously sampled by M cells within Peyer's patches. TSLP produced by TLR signaling in the epithelium enhances production of APRIL and BAFF by DC. Additionally retinoic acid produced by mucosal DC appears to determine the specificity of IgA class switch recombination in the mucosa. Activated T cells may also provide cognate help for T cell-dependent switching to IgA, although a significant proportion of IgA class-switching is T cell-independent.
Epithelial Regulation of DC Functions and Oral Tolerance
In mice and humans, the intestinal DC are located throughout intestinal LP and in the radial muscle layer (58). They also accumulate in the lymphoid tissues of the mucosa, namely PP, ILF, and MLN (59). DC are the most important professional antigen-presenting cells and express up to 100 times more MHC and are more effective at differentiating naïve T cells than other APCs (60, 61). In the PP and ILF DC are considered to be primarily responsible for T cell-dependent IgA responses (62). In the LP, two major subsets of DC can be discriminated that perform different immune functions. The LP, CD103+ (also known as αEβ7 integrin) expressing DC have recently been shown to play a key role in regulating oral tolerance through the induction of regulatory Foxp3+ T cells expressing gut-homing receptors in the MLN (Fig. 3) (63, 64). A subset of LP CD103− cells which share phenotypic traits with both macrophages and DC express the fractalkine receptor (CX3CR1) and bind to the membrane form of fractalkine on IEC (65, 66). The ability of DC to produce extensions and sample antigens through the epithelium is reported to depend on CX3CR1 expression (67), although this is somewhat controversial, and it is possible that other subsets of DC can sample antigens in this way (46). Recently, the CD103− CX3CR1+ subset of LP DC were shown to be derived from a different cell linage to the CD103+ population (68) and seem to support inflammatory immune responses (69, 70). Taken together these findings suggest that the mucosal CD103+ DC serve classical DC functions and promote T cell responses in the draining lymph nodes, whereas the CX3CR1+ DC are retained in the LP to kill colonizing and invading microorganisms and induce inflammatory responses.
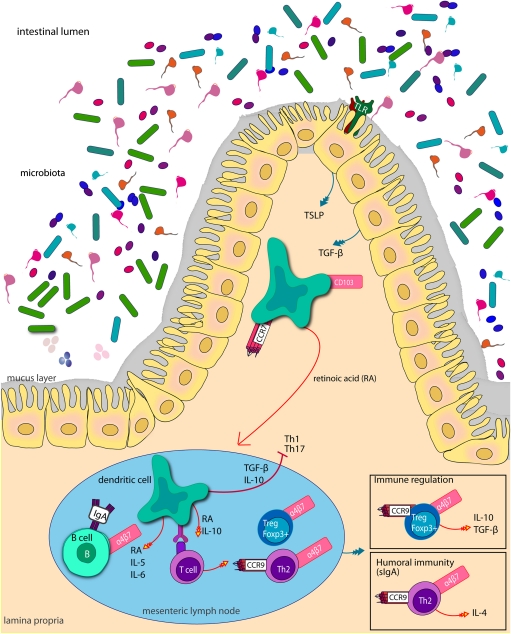
Fig. 3.
Mucosal CD103+ DC are conditioned in the peripheral tissues by epithelial production of TSLP and TGF-β, which endows DC with the ability to prime noninflammatory responses and induce regulatory T cells. When they receive an inflammatory or danger signal, they begin to mature, and the expression of CCR7 increases allowing the DC to enter lymph vessels and migrate to the draining lymph nodes. In the T cell areas retinoic acid, the acid form of vitamin A plays an important role in the ability of DC to up-regulate homing receptors on lymphocytes. Retinoic acid (RA) is also an important cofactor for the differentiation of Foxp3+ Tregs and has been shown to inhibit the generation of Th17 cells.
Several studies have implicated a role for epithelial cells in endowing DC with their ability to prime noninflammatory responses and induce regulatory T cells (64, 71, 72). Conditioning of monocyte-derived DC with IEC supernatants in vitro abolishes the ability of DC to produce IL-12 and prime naïve T cells toward Th1 polarization in response to microbial stimuli (71). In contrast, EC-conditioned DC produce high amounts of IL-10 and promote Treg and Th2 cell responses. In vitro the conditioning of DC was shown to depend on epithelial production of TSLP (Fig. 3) (71). Another important immunoregulatory cytokine produced abundantly by IEC and stromal cells in the intestine is TGF-β (72). This cytokine inhibits NF-κB-dependent gene expression and the production of proinflammatory cytokines by macrophages and DC (73, 74). Additionally, TGF-β acts in concert with TSLP to induce a tolerogenic phenotype in monocyte-derived DC in vitro (75). DC purified from the LP have also been shown to promote a high level of regulatory T (Treg) cell conversion relative to lymphoid organ-derived DC via a TGF-β and retinoic acid-dependent mechanism. Consistent with these findings is the fact that TSLP deletion in mice leads to constitutive overexpression of IL-12p40 by intestinal DC and inability to generate protective regulatory and Th2 responses against the nematode parasite Trichuris muris (76).
TSLP mRNA is constitutively expressed by epithelial cells and can be up-regulated by NF-κB-dependent pathways (77). Thus, one may expect that recognition of microbiota by epithelial PRR would also regulate TSLP production. Support for this idea comes from in vitro IEC-DC co-culture studies where it was shown that composition of the microbiota exposed to the apical side of the IEC influenced production of TSLP and TGF-β and, hence, the function of the underlying DC (75). In an in vivo expression profiling study where healthy adult humans consumed preparations of viable lactic acid bacteria, a central role was uncovered for the NF-κB signaling cascade in the regulation of tolerance in the small intestine (78). In this study, it was found that NF-κB signaling up-regulated the expression of downstream effectors such as chemokines but also factors that regulate cell survival of B and T cells and DC as well as regulators that suppress inappropriate immune responses.
In addition to the epithelial cytokines influencing B cell and DC functions mentioned above, the intestinal epithelium expresses a range of metabolic enzymes that can impact on immune cell function. Non-bone marrow-derived stromal cells located predominantly in the villi of proximal small intestine have been shown to constitutively produce cyclooxygenase (COX)-2 and abundantly produce the COX-2-dependent arachidonic acid (AA) metabolite, prostaglandin E2 (PGE2) (79). Although the production of COX-2 and COX-2-dependent metabolites does not appear to be regulated by proinflammatory stimuli or the microbiota, its production in the epithelium could contribute to the default immunoregulatory tone of the LP (80).
Conclusions
Endocrine, goblet cells, and enterocytes of the intestinal epithelium express a range of PRR to sense the presence of microbes. The best characterized are the TLR and NOD receptors, which are well known for their roles in pathogen recognition and the induction of innate effectors and inflammation (17). The innate barrier functions of the epithelium play an important role in maintaining a peaceful relationship with the commensal community of gut bacteria (4). These innate effectors are regulated by PRR signaling which explains why mice with specific defects in NF-κB pathway or TLR signaling, are more susceptible than normal mice to the development of colitis (27, 29). Additionally, the production of sIgA antibodies to the microbiota limits epithelial contact and invasion of host cells. Epithelial cells produce APRIL and BAFF, which promote B cell recruitment in the LP and class switching in response to TLR signaling. Thus, the host recognition of intestinal microbes is inextricably linked to the production of sIgA and the immune exclusion of microbes (57). Despite the existence of several mechanisms to avoid intimate contact of the epithelium with intestinal bacteria, the LP has a distinctly immunosuppressive tone to inhibit over reaction to innocuous luminal antigens including the commensal microbiota. This mechanism of “oral tolerance” depends largely on the development of Treg cells in the draining lymph nodes. Epithelial cells produce TSLP and TGF-β and possibly other factors that abolish the ability of DC to produce inflammatory cytokine responses and promote the induction of Treg cells in the MLN (71). TSLP is up-regulated by NF-κB-dependent pathways, suggesting that PRR signaling from the luminal side of the epithelium would enhance the suppressive tone in the gut, normally keeping inflammation under control. In the case of infection however, chemokines secreted by epithelial cells would recruit unconditioned DC to mucosal sites, which deviates the response to a more proinflammatory character.
The identification of microbe–IEC interactions as having a crucial role in the regulation of several mucosal immunological functions will encourage future efforts to unravel the molecular mechanisms and cellular pathways involved. Ultimately, a better understanding of the host–microbe interactions in the gut will provide new opportunities for the prevention and treatment of a number of inflammatory disorders associated with the aging population and Westernized societies (81, 82).
Acknowledgments
O.R. is a Marie Curie Research Fellow in the EC FP7 Cross-talk project (PITN-GA-2008-215553) and gratefully acknowledges their financial support.
Footnotes
The authors declare no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Microbes and Health” held November 2–3, 2009, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at http://www.nasonline.org/SACKLER_Microbes_and_Health.
This article is a PNAS Direct Submission.
References
- 1.Gill SR, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qin J, et al. MetaHIT Consortium. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salzman NH, Underwood MA, Bevins CL. Paneth cells, defensins, and the commensal microbiota: A hypothesis on intimate interplay at the intestinal mucosa. Semin Immunol. 2007;19:70–83. doi: 10.1016/j.smim.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Johansson ME, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Si-Tahar M, Merlin D, Sitaraman S, Madara JL. Constitutive and regulated secretion of secretory leukocyte proteinase inhibitor by human intestinal epithelial cells. Gastroenterology. 2000;118:1061–1071. doi: 10.1016/s0016-5085(00)70359-3. [DOI] [PubMed] [Google Scholar]
- 7.Eckmann L, et al. Differential cytokine expression by human intestinal epithelial cell lines: Regulated expression of interleukin 8. Gastroenterology. 1993;105:1689–1697. doi: 10.1016/0016-5085(93)91064-o. [DOI] [PubMed] [Google Scholar]
- 8.Lundqvist C, Melgar S, Yeung MM, Hammarström S, Hammarström ML. Intraepithelial lymphocytes in human gut have lytic potential and a cytokine profile that suggest T helper 1 and cytotoxic functions. J Immunol. 1996;157:1926–1934. [PubMed] [Google Scholar]
- 9.Li Y, et al. Disease-related expression of the IL-6/STAT3/SOCS3 signaling pathway in ulcerative colitis and ulcerative colitis-related carcinogenesis. Gut. 2009;59:227–235. doi: 10.1136/gut.2009.184176. [DOI] [PubMed] [Google Scholar]
- 10.Rehwinkel J, Reis e Sousa C. RIGorous detection: Exposing virus through RNA sensing. Science. 2010;327:284–286. doi: 10.1126/science.1185068. [DOI] [PubMed] [Google Scholar]
- 11.Williams A, Flavell RA, Eisenbarth SC. The role of NOD-like receptors in shaping adaptive immunity. Curr Opin Immunol. 2010;22:34–40. doi: 10.1016/j.coi.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inohara N, et al. Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-kappaB. J Biol Chem. 1999;274:14560–14567. doi: 10.1074/jbc.274.21.14560. [DOI] [PubMed] [Google Scholar]
- 13.Hisamatsu T, Suzuki M, Podolsky DK. Interferon-gamma augments CARD4/NOD1 gene and protein expression through interferon regulatory factor-1 in intestinal epithelial cells. J Biol Chem. 2003;278:32962–32968. doi: 10.1074/jbc.M304355200. [DOI] [PubMed] [Google Scholar]
- 14.Kim JG, Lee SJ, Kagnoff MF. Nod1 is an essential signal transducer in intestinal epithelial cells infected with bacteria that avoid recognition by toll-like receptors. Infect Immun. 2004;72:1487–1495. doi: 10.1128/IAI.72.3.1487-1495.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lala S, et al. Crohn's disease and the NOD2 gene: A role for paneth cells. Gastroenterology. 2003;125:47–57. doi: 10.1016/s0016-5085(03)00661-9. [DOI] [PubMed] [Google Scholar]
- 16.Gutierrez O, et al. Induction of Nod2 in myelomonocytic and intestinal epithelial cells via nuclear factor-kappa B activation. J Biol Chem. 2002;277:41701–41705. doi: 10.1074/jbc.M206473200. [DOI] [PubMed] [Google Scholar]
- 17.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: How bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 18.Wells JM, Loonen LM, Karczewski JM. The role of innate signaling in the homeostasis of tolerance and immunity in the intestine. Int J Med Microbiol. 2010;300:41–48. doi: 10.1016/j.ijmm.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Furrie E, Macfarlane S, Thomson G, Macfarlane GT, Microbiology & Gut Biology Group, Tayside Tissue & Tumour Bank Toll-like receptors-2, -3 and -4 expression patterns on human colon and their regulation by mucosal-associated bacteria. Immunology. 2005;115:565–574. doi: 10.1111/j.1365-2567.2005.02200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cario E, et al. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 21.Abreu MT, et al. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol. 2001;167:1609–1616. doi: 10.4049/jimmunol.167.3.1609. [DOI] [PubMed] [Google Scholar]
- 22.Otte JM, Podolsky DK. Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am J Physiol Gastrointest Liver Physiol. 2004;286:G613–G626. doi: 10.1152/ajpgi.00341.2003. [DOI] [PubMed] [Google Scholar]
- 23.Ortega-Cava CF, et al. Strategic compartmentalization of Toll-like receptor 4 in the mouse gut. J Immunol. 2003;170:3977–3985. doi: 10.4049/jimmunol.170.8.3977. [DOI] [PubMed] [Google Scholar]
- 24.Chabot S, Wagner JS, Farrant S, Neutra MR. TLRs regulate the gatekeeping functions of the intestinal follicle-associated epithelium. J Immunol. 2006;176:4275–4283. doi: 10.4049/jimmunol.176.7.4275. [DOI] [PubMed] [Google Scholar]
- 25.Hornef MW, Frisan T, Vandewalle A, Normark S, Richter-Dahlfors A. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J Exp Med. 2002;195:559–570. doi: 10.1084/jem.20011788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hornef MW, Normark BH, Vandewalle A, Normark S. Intracellular recognition of lipopolysaccharide by toll-like receptor 4 in intestinal epithelial cells. J Exp Med. 2003;198:1225–1235. doi: 10.1084/jem.20022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J, et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol. 2006;8:1327–1336. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- 28.Shibolet O, Podolsky DK. TLRs in the Gut. IV. Negative regulation of Toll-like receptors and intestinal homeostasis: Addition by subtraction. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1469–G1473. doi: 10.1152/ajpgi.00531.2006. [DOI] [PubMed] [Google Scholar]
- 29.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Schilling JD, Martin SM, Hung CS, Lorenz RG, Hultgren SJ. Toll-like receptor 4 on stromal and hematopoietic cells mediates innate resistance to uropathogenic Escherichia coli. Proc Natl Acad Sci USA. 2003;100:4203–4208. doi: 10.1073/pnas.0736473100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandl K, Plitas G, Schnabl B, DeMatteo RP, Pamer EG. MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection. J Exp Med. 2007;204:1891–1900. doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lebeis SL, Bommarius B, Parkos CA, Sherman MA, Kalman D. TLR signaling mediated by MyD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium. J Immunol. 2007;179:566–577. doi: 10.4049/jimmunol.179.1.566. [DOI] [PubMed] [Google Scholar]
- 33.Hugot JP, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 34.Maeda S, et al. Nod2 mutation in Crohn's disease potentiates NF-kappaB activity and IL-1beta processing. Science. 2005;307:734–738. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- 35.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vora P, et al. Beta-defensin-2 expression is regulated by TLR signaling in intestinal epithelial cells. J Immunol. 2004;173:5398–5405. doi: 10.4049/jimmunol.173.9.5398. [DOI] [PubMed] [Google Scholar]
- 37.Biragyn A, et al. DNA vaccines encoding human immunodeficiency virus-1 glycoprotein 120 fusions with proinflammatory chemoattractants induce systemic and mucosal immune responses. Blood. 2002;100:1153–1159. doi: 10.1182/blood-2002-01-0086. [DOI] [PubMed] [Google Scholar]
- 38.Yang D, et al. Beta-defensins: Linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 39.Vongsa RA, Zimmerman NP, Dwinell MB. CCR6 regulation of the actin cytoskeleton orchestrates human beta defensin-2- and CCL20-mediated restitution of colonic epithelial cells. J Biol Chem. 2009;284:10034–10045. doi: 10.1074/jbc.M805289200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Funderburg N, et al. Human -defensin-3 activates professional antigen-presenting cells via Toll-like receptors 1 and 2. Proc Natl Acad Sci USA. 2007;104:18631–18635. doi: 10.1073/PNAS.0702130104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johansen FE, et al. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J Exp Med. 1999;190:915–922. doi: 10.1084/jem.190.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 43.Hanson LA. Comparative analysis of human milk and human blood plasma by means of diffusion-in-gel methods. Experientia. 1959;15:473–474. doi: 10.1007/BF02158262. [DOI] [PubMed] [Google Scholar]
- 44.Abreu MT, et al. TLR signaling at the intestinal epithelial interface. J Endotoxin Res. 2003;9:322–330. doi: 10.1179/096805103225002593. [DOI] [PubMed] [Google Scholar]
- 45.Rescigno M, Rotta G, Valzasina B, Ricciardi-Castagnoli P. Dendritic cells shuttle microbes across gut epithelial monolayers. Immunobiology. 2001;204:572–581. doi: 10.1078/0171-2985-00094. [DOI] [PubMed] [Google Scholar]
- 46.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brandtzaeg P, et al. The B-cell system of human mucosae and exocrine glands. Immunol Rev. 1999;171:45–87. doi: 10.1111/j.1600-065X.1999.tb01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Owens WE, Berg RD. Bacterial translocation from the gastrointestinal tract of athymic (nu/nu) mice. Infect Immun. 1980;27:461–467. doi: 10.1128/iai.27.2.461-467.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol. 1996;4:430–435. doi: 10.1016/0966-842x(96)10057-3. [DOI] [PubMed] [Google Scholar]
- 50.Peterson DA, McNulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2:328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 51.Bekeredjian-Ding I, Jego G. Toll-like receptors—sentries in the B-cell response. Immunology. 2009;128:311–323. doi: 10.1111/j.1365-2567.2009.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mora JR, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 53.Renegar KB, Small PA., Jr Immunoglobulin A mediation of murine nasal anti-influenza virus immunity. J Virol. 1991;65:2146–2148. doi: 10.1128/jvi.65.4.2146-2148.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dignass AU, Podolsky DK. Cytokine modulation of intestinal epithelial cell restitution: Central role of transforming growth factor beta. Gastroenterology. 1993;105:1323–1332. doi: 10.1016/0016-5085(93)90136-z. [DOI] [PubMed] [Google Scholar]
- 55.Brown SL, et al. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Invest. 2007;117:258–269. doi: 10.1172/JCI29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shang L, et al. Toll-like receptor signaling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. Gastroenterology. 2008;135:529–538. doi: 10.1053/j.gastro.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He B, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 58.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johansson C, Kelsall BL. Phenotype and function of intestinal dendritic cells. Semin Immunol. 2005;17:284–294. doi: 10.1016/j.smim.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 60.Inaba K. Dendritic cells as antigen-presenting cells in vivo. Immunol Cell Biol. 1997;75:206–208. doi: 10.1038/icb.1997.31. [DOI] [PubMed] [Google Scholar]
- 61.Levin D, Constant S, Pasqualini T, Flavell R, Bottomly K. Role of dendritic cells in the priming of CD4+ T lymphocytes to peptide antigen in vivo. J Immunol. 1993;151:6742–6750. [PubMed] [Google Scholar]
- 62.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 63.Jaensson E, et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iliev ID, et al. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut. 2009;58:1481–1489. doi: 10.1136/gut.2008.175166. [DOI] [PubMed] [Google Scholar]
- 65.Mora JR, et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 66.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Niess JH, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 68.Bogunovic M, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Niess JH, Adler G. Enteric flora expands gut lamina propria CX3CR1+ dendritic cells supporting inflammatory immune responses under normal and inflammatory conditions. J Immunol. 2010;184:2026–2037. doi: 10.4049/jimmunol.0901936. [DOI] [PubMed] [Google Scholar]
- 70.Varol C, et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 71.Rimoldi M, et al. Monocyte-derived dendritic cells activated by bacteria or by bacteria-stimulated epithelial cells are functionally different. Blood. 2005;106:2818–2826. doi: 10.1182/blood-2004-11-4321. [DOI] [PubMed] [Google Scholar]
- 72.Jarry A, et al. Mucosal IL-10 and TGF-beta play crucial roles in preventing LPS-driven, IFN-gamma-mediated epithelial damage in human colon explants. J Clin Invest. 2008;118:1132–1142. doi: 10.1172/JCI32140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaliński P, Vieira PL, Schuitemaker JH, de Jong EC, Kapsenberg ML. Prostaglandin E(2) is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood. 2001;97:3466–3469. doi: 10.1182/blood.v97.11.3466. [DOI] [PubMed] [Google Scholar]
- 74.Smythies LE, et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zeuthen LH, Fink LN, Frokiaer H. Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-beta. Immunology. 2008;123:197–208. doi: 10.1111/j.1365-2567.2007.02687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zaph C, et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 77.Allakhverdi Z, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van Baarlen P, et al. Differential NF-kappaB pathways induction by Lactobacillus plantarum in the duodenum of healthy humans correlating with immune tolerance. Proc Natl Acad Sci USA. 2009;106:2371–2376. doi: 10.1073/pnas.0809919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Newberry RD, McDonough JS, Stenson WF, Lorenz RG. Spontaneous and continuous cyclooxygenase-2-dependent prostaglandin E2 production by stromal cells in the murine small intestine lamina propria: Directing the tone of the intestinal immune response. J Immunol. 2001;166:4465–4472. doi: 10.4049/jimmunol.166.7.4465. [DOI] [PubMed] [Google Scholar]
- 80.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 81.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 82.Sicherer SH, Sampson HA. Food allergy: Recent advances in pathophysiology and treatment. Annu Rev Med. 2009;60:261–277. doi: 10.1146/annurev.med.60.042407.205711. [DOI] [PubMed] [Google Scholar]