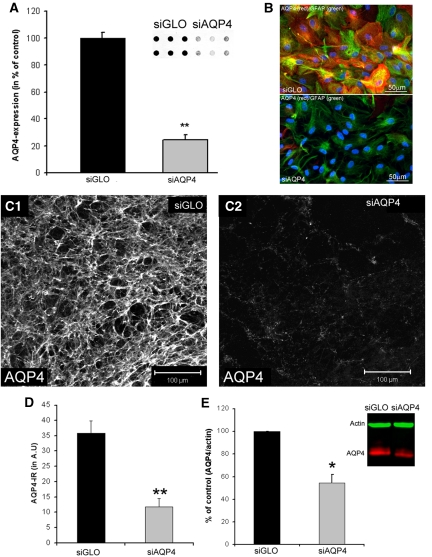
Figure 2.
In vitro application of small interference RNA against AQP4 (siAQP4) decreases AQP4 expression. (A) AQP4 expression was quantified from dot blot experiments (inset) in primary astrocyte cultures treated with siGLO (control) and siAQP4. AQP4 expression was decreased 3 days after siAQP4 application (76%±4%, **t-test, P<0.001, n=6). (B) AQP4 (red) and GFAP (green) immunolabeling in primary astrocyte cultures treated with siGLO (control, n=6) and siAQP4 (n=6) showed no morphological differences between groups. Nuclei were stained with DAPI (blue). (C) Confocal images of AQP4 immunoreactivity (AQP4-ir) in CA1 of control (siGLO, C1) and siAQP4-treated organotypic hippocampal slices (C2) demonstrated a large decrease in AQP4 staining. (D) Optical densities of immunohistochemistry from organotypic slices showed a significant decrease in AQP4-ir from siAQP4-treated slices (67%) compared with siGLO-treated slices (analysis of variance and Tukey–Kramer multiple comparisons tests, **P<0.001, n=8). These results provided the basis for our small interference RNA (siRNA) transfection protocol for in vivo experiments. (E) AQP4 expression quantified from Western blot experiments (inset) in organotypic hippocampal slice cultures treated with siGLO (control), or siAQP4. Treatment with siAQP4 decreased AQP4 levels (inset: red) (54%±7%) compared with siGLO-treated (i.e., control) hippocampal slices (Kruskal–Wallis test, nonparametric analysis of variance, *P<0.05, n=6). GFAP, glial fibrillary acid protein.