Abstract
Nearly 15 million people in the United States suffer from either aortic or mitral valvular disease. For patients with severe and symptomatic valvular heart disease, valve replacement surgery improves morbidity and mortality outcomes. In 2009, 90,000 valve replacement surgeries were performed in the United States. This review evaluates the advantages and disadvantages of mechanical and bioprosthetic prosthetic heart valves as well as the factors for consideration in deciding the appropriate valve type for an individual patient. Although many caveats exist, the general recommendation is for patients younger than 60 to 65 years to receive mechanical valves due to the valve’s longer durability and for patients older than 60 to 65 years to receive a bioprosthetic valve to avoid complications with anticoagulants. Situations that warrant special consideration include patient co-morbidities, the need for anticoagulation, and the potential for pregnancy. Once these characteristics have been considered, patients’ values, anxieties, and expectations for their lifestyle and quality of life should be incorporated into final valve selection. Decision aids can be useful in integrating preferences in the valve decision. Finally, future directions in valve technology, anticoagulation, and medical decision-making are discussed.
Keywords: prosthetic heart valves, patient preference, valve type, anticoagulant, structural valve deterioration
Overview
Nearly 15 million people in the United States suffer from either aortic or mitral valvular disease.1,2 For patients with severe and symptomatic valvular heart disease, valve replacement surgery improves morbidity and mortality outcomes. In 2009, 90,000 valve replacement surgeries were performed in the United States.3 In general, 2 options exist for replacement valves – mechanical and bioprosthetic valves. The decision on which valve replacement to use requires careful consideration of the specific advantages and disadvantages of the valve types and integration of this knowledge into the clinical characteristics and personal preferences of the individual patient. This article outlines advantages and disadvantages of each valve type, as well as outcomes and factors for consideration in deciding the appropriate valve type for an individual patient.
Mechanical valves
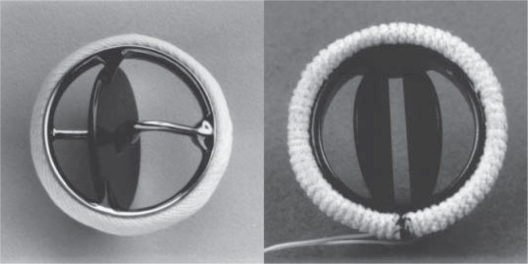
The two common types of mechanical valves, tilting-disc and bileaflet valves (Figure 1),4 have comparable durability and both require life-long anticoagulation therapy due to their associated thrombotic risk.5
Figure 1.
Photographs of commonly used prosthetic valves. Left: single-tilting-disk (Medtronic-Hall, Medtronic, Minneapolis, MN) valve; Right: bileaflet-tilting-disk (St Jude Medical, Little Canada, MN) valve.
Notes: Copyright © 1996. Massachusetts Medical Society. Reprinted with permission from Vongpatanasin W, Hillis LD, Lange RA. Medical progress: prosthetic heart valves. N Engl J Med. 1996;335:407–416.4
Mechanical valve advantages
Mechanical valves have advantages and disadvantages, the most important of which is their greater durability (20–30 years) than tissue bioprosthetic valves (10–15 years).6–9 Their greater durability translates into lower reoperation rates among these patients, compared with patients with bioprosthetic valves.9,10
The excellent durability of mechanical valves was illustrated most definitively by a trial that randomized 575 patients between 1977 and 1982 at 13 different US Department of Veterans Affairs centers to receive either a mechanical or bioprosthetic valve replacement. The investigators found that patients younger than 65 years who received a bioprosthetic valve had a greater rate of primary valve failure for both aortic valve replacements (AVR) and mitral valve replacements (MVR) 15 years after implantation compared with similarly aged patients with mechanical valve replacements (bioprosthetic vs mechanical 26% vs 0%, P < 0.001 for AVR and 44% vs 4%, P < 0.001 for MVR). However, in patients older than 65, there was no significant difference in primary valve failure between the two valve types, presumably due to the shorter lifespans of the older patients.11 This large randomized control study demonstrates the excellent durability of mechanical heart valves compared with bioprosthetic heart valves.
Mechanical valve disadvantages
Although mechanical valves are more durable than bioprosthetic valves, mechanical valves also have several disadvantages that a provider and patient must consider. Blood flow around the mechanical valve results in high sheer stresses, which can result in platelet activation and a higher risk for thrombosis on the valve surface and a subsequent risk for embolism. Given this risk, all patients with mechanical heart valves require lifelong anticoagulation, most commonly with a vitamin K antagonist such as warfarin. Although warfarin use is efficacious in reducing thrombosis risk, it heightens hemorrhagic risk.9,10,12,13 For example, a 60-year-old male with a mechanical valve replacement has a lifetime risk of bleeding of 41% compared with a 12% risk in a similar patient with a bioprosthetic valve replacement.14
Anticoagulation and bleeding
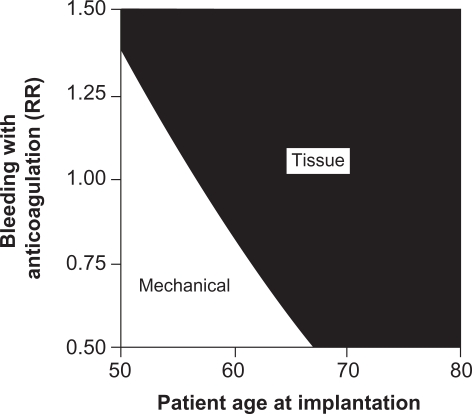
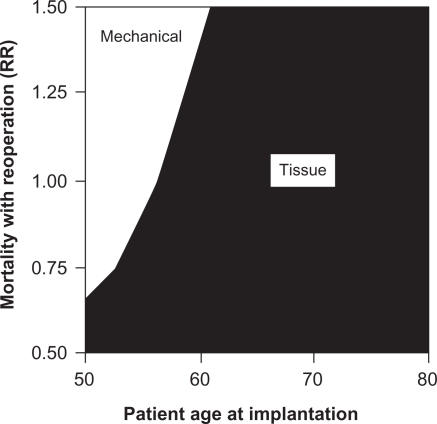
Furthermore, the risk of bleeding from anticoagulant therapy increases as patients age.15–25 Patients with mechanical valves on anticoagulation therapy who are older than 60 years are nearly 7 times more likely to bleed than patients younger than 60.15 The increased risk of bleeding with a mechanical valve replacement in older patients further supports avoiding mechanical valves in this population. Figure 2 illustrates this concept in a 2-way sensitivity analysis that studies the interaction between age and bleeding risk in deciding on valve type. As the age of the patient increases and/or the relative risk of bleeding on anticoagulation increases, tissue valve implantation becomes more favorable than mechanical valve implantation.26
Figure 2.
Two-way sensitivity analysis of the effects of anticoagulant-related bleeding and patient age at implantation on the recommended valve type.
Notes: RR = relative risk where RR = 1 is the baseline estimate, RR = 1.5 is 50% higher than the baseline estimate, and RR = 0.5 is 50% lower than the baseline estimate. Copyright © 2000. Elsevier. Reprinted with permission from Birkmeyer NJ, Birkmeyer JD, Tosteson AN, et al. Prosthetic valve type for patients undergoing aortic valve replacement: a decision analysis. Ann Thorac Surg. 2000;70:1946–1952.26
The need for anticoagulation therapy, usually with warfarin, introduces a variety of additional considerations for both providers and patients. Therapeutic levels of warfarin are difficult to achieve and maintain, due to both barriers to adherence and the variety of interactions that warfarin has with other medications and diet. A recent study underscored this difficulty by demonstrating that only 62% of those patients with a mechanical valve on anticoagulation medication are found within the appropriate international normalized ratio (INR) range, even in the setting of adequate medication adherence.15 In order to maximize the benefits and minimize the risks of warfarin therapy, patients are required to make significant and lifelong lifestyle adjustments, including frequent office visits to monitor INR levels, monitoring of their diet to maintain consistent levels of vitamin K, and avoidance of contact sports and other potentially traumatic situations.26
Anticoagulation and co-morbidities
Individual patient co-morbidities also affect the decision for mechanical valve replacement and its attendant need for anticoagulation. For example, a mechanical valve is recommended when the patient is already on anticoagulants for another medical condition, such as atrial fibrillation or a thrombotic disorder.3,27 Patients and providers also need to consider future scenarios where discontinuation of anticoagulation with warfarin would be necessary, such as surgical procedures or pregnancy.28 Warfarin is contraindicated with pregnancy. As such, the provider and patient need to consider the implantation of a bioprosthetic valve in a woman with the potential to become pregnant. Careful discussion of these possibilities and ongoing communication between provider and patient are essential to anticipate these events and adjust therapies accordingly.
In summary, mechanical valves offer greater durability at the expense of lifelong anticoagulation, higher bleeding risks, and the attendant lifestyle modifications and considerations to minimize these risks.3,29,30 Accordingly, mechanical valves are generally recommended for younger patients since a patient with a longer life expectancy is more likely to outlive a bioprosthetic valve and require a reoperation.13,26,31 However, valve type selection should be a shared decision-making process in choosing the optimal prosthetic heart valve for a specific patient in order to minimize the valve’s associated risks.3,13,26,29–31
Bioprosthetic valves
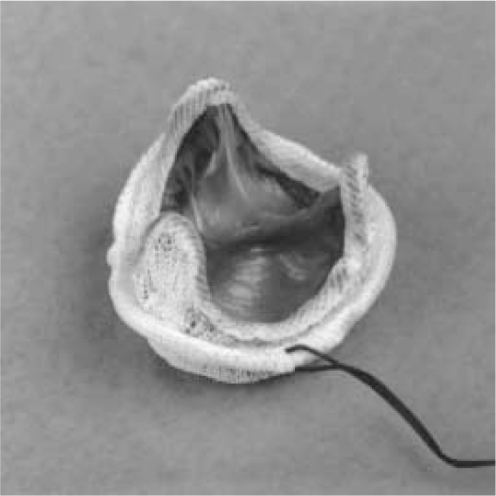
Bioprosthetic valves commonly used in clinical practice are called heterografts, which are usually stented or stentless porcine or bovine tissue valves3 (see Figure 3).4 Heterografts have similar advantages and limitations relative to mechanical valve replacements.32
Figure 3.
Photograph of Porcine (Carpentier–Edwards) Bioprosthesis.
Notes: Copyright © 1996. Massachusetts Medical Society. Reprinted with permission from Vongpatanasin W, Hillis LD, Lange RA. Medical progress: prosthetic heart valves. N Engl J Med. 1996;335:407–416.4
Bioprosthetic valve advantages
The main advantage with bioprosthetic valves is that they do not require lifelong warfarin therapy, due to their lower thrombotic risk compared with mechanical valves (0.87% and 1.4%, per year respectively).13,14 Accordingly, patients with bioprosthetic valves have a significantly decreased risk of bleeding.10,14
Bioprosthetic valve disadvantages
The bioprosthetic valve also has disadvantages. Whereas the mechanical valve has an increased risk of thromboembolism but is more durable, the prosthetic valve has a decreased risk of thromboembolism but is less durable. This process of structural valve deterioration is poorly understood but is thought to result from the accumulation of calcium and lipids on the valve surface.33 An incomplete saline rinse of the valve both prior to and during surgery can also exacerbate structural valve deterioration leading to an increased risk of valve thickening and calcification.34 Improvements in second-generation bioprosthetic valves have reduced the rapidity of deterioration compared with first-generation valves, but structural valve deterioration remains a major disadvantage for bioprosthetic valves.10 For most patients with a bioprosthetic valve, structural valve deterioration begins around 5 years post-implantation and rapidly increases. For example, one study demonstrated that at 5 years post-implantation of a bioprosthetic valve, structural valve deterioration occurred in 1.0% ± 0.3% of patients. At 10 and 15 years post-implantation, rates of structural valve deterioration increased to 17.2% ± 2.2% and 37.2% ± 5.8% of patients, respectively.13
Bioprosthetic valves and reoperation
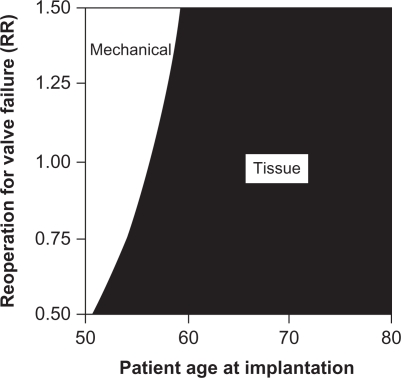
This increased risk of deterioration translates to an increased risk of reoperation for these patients. One study compared the durability of the bioprosthetic valve and mechanical valve and concluded that the lifetime risk of reoperation is 25% for a bioprosthetic valve compared with 3% for a mechanical valve.10,14 This risk of reoperation is a major driver in the decision to select a mechanical or tissue valve. Figure 4 illustrates this concept with a 2-way sensitivity analysis comparing the relative risk for reoperation to the patient’s age at initial valve implantation. As the relative risk of reoperation increases, the optimal decision favors mechanical valve implantation for more patients. However, at age 60 years and higher, tissue valves appear to be the dominant strategy even with a 50% higher risk of reoperation, since the expected lifespan of the patient is not likely to exceed the lifespan of the tissue valve.26
Figure 4.
Two-way sensitivity analysis of the effects of reoperation for tissue valve failure and patient age at implantation on the recommended valve type.
Notes: RR = relative risk where RR = 1 is the baseline estimate, RR = 1.5 is 50% higher than the baseline estimate, and RR = 0.5 is 50% lower than the baseline estimate. Copyright © 2000. Elsevier. Reprinted with permission from Birkmeyer NJ, Birkmeyer JD, Tosteson AN, et al. Prosthetic valve type for patients undergoing aortic valve replacement: a decision analysis. Ann Thorac Surg. 2000;70:1946–1952.26
Reoperation to replace a failed prosthetic valve has risks. A 2009 analysis from a Vancouver dataset demonstrated that the mortality risk of reoperation to replace a bioprosthetic valve that has deteriorated is 7.3% (average patient age was 54 years old).14 Interestingly, old age does not excessively increase mortality in valve replacement surgeries.35 Nonetheless, proper decision-making around valve replacement requires consideration of the risks of reoperation and its associated mortality. Figure 5 demonstrates this trade-off in a 2-way sensitivity analysis comparing the relative risk of reoperation-associated mortality and patient age at valve implantation.26 At younger ages, higher relative risks of surgical mortality favor implantation of a mechanical valve. However, for patients aged 60 years or more, the likelihood of reoperation is low enough to justify tissue valve implantation, even in patients with a 50% higher relative risk of reoperative mortality.
Figure 5.
Two-way sensitivity analysis of the effects of mortality with reoperation and patient age at implantation on the recommended valve type.
Notes: RR = relative risk where RR = 1 is the baseline estimate, RR = 1.5 is 50% higher than the baseline estimate, and RR = 0.5 is 50% lower than the baseline estimate. Copyright © 2000. Elsevier, Reprinted with permission from Birkmeyer NJ, Birkmeyer JD, Tosteson AN, et al. Prosthetic valve type for patients undergoing aortic valve replacement: a decision analysis. Ann Thorac Surg. 2000;70:1946–1952.26
Given these relative risks of structural valve deterioration and subsequent reoperation, the current general recommendation for patients older than 60 to 65 years is a bioprosthetic valve and for patients less than 60 to 65 years is a mechanical valve.10,26 For a 65-year-old patient, structural valve deterioration of a tissue valve will occur, on average, 10 to 15 years after implantation while the patient’s life expectancy is only 11.3 years.10,36 Thus, a patient choosing to undergo a prosthetic valve replacement at 65 years will have a 28% chance of needing a reoperation.10 As tissue valves continue to improve in durability and rates of reoperation decrease, lowering of the recommended age for tissue valve implantation may occur.11,15,36,37 Conversely, as life expectancy continues to increase, more elderly patients may face the need for a tissue valve replacement. Thus, decision-making about valve type will need to keep these changes in mind.
Bioprosthetic valves and co-morbidities
Certain subpopulations of patients require special consideration in the decision to implant a tissue valve. Co-morbidities that confer a shortened life expectancy, such as renal failure requiring dialysis or cancer, may favor tissue valve implantation.3,15 Other co-morbidities, such as hyperparathyroidism, appear to accelerate tissue valve deterioration and thus may favor mechanical valve implantation.3 Accordingly, the general recommendations for valve selection need to be considered in light of the patient’s co-morbidities and individual circumstances.
Factors affecting valve selection
The process of deciding which prosthetic valve type is best for an individual patient is complex. Consideration of the general advantages and disadvantages of the valve types, as outlined above, is only the first step. These general recommendations need to be then tailored to the individual patient’s clinical condition and, equally importantly, personal preferences. Once the specific advantages and disadvantages are considered in the context of the individual patient, then the information needs to be communicated in an effective way for the provider and patient to fully comprehend the consequences of valve selection. Finally, frameworks to allow for incorporation of the myriad factors to aid decision-making are necessary.
Age
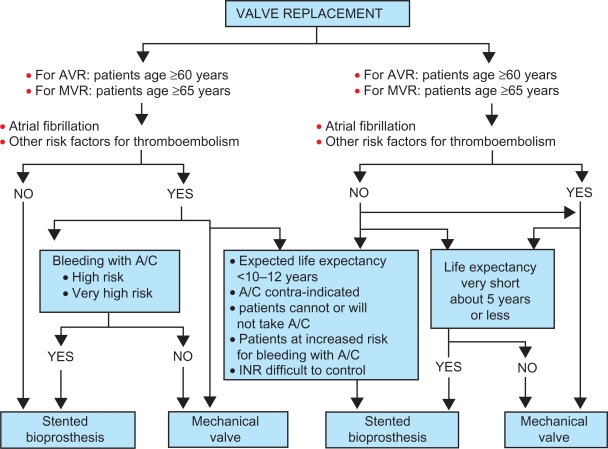
The general recommendations around valve choice center on age, taking into consideration the life expectancy of the patient in relation to the valve. Patients older than 65 years typically do not outlive the life expectancy of a tissue valve.11,15 In light of the higher bleeding event rates relative to reoperation rates, implanting a tissue valve in an elderly patients can avoid anticoagulation and its attendant bleeding risks.3,10 Thus, the current recommendation for patients older than 60 to 65 years is a bioprosthetic valve. Patients younger than 50 years are more likely to experience structural valve deterioration and a need for reoperation; so mechanical valves are generally recommended for younger patients. Figure 6 is an example of an algorithm that integrates general recommendations with patient co-morbidities, valve risk assessment, life expectancy, and patient preference.15
Figure 6.
Algorithm for choice of prosthetic heart valve.
Notes: Copyright © 2010. Elsevier. Reprinted with permission from Rahimtoola SH. Choice of prosthetic heart valve in adults: an update. J Am Coll Cardiol. 2010;55: 2413–2426.15
Abbreviations: A/C, anticoagulants; AVR, aortic valve replacements; INR, international normalized ratio; MVR, mitral valve replacements.
In support of current recommendations, studies have compared the lifetime rates of reoperation after bioprosthetic valve implantation to the rates of bleeding events after mechanical valve implantation and concurrent anticoagulation.10 Among 35-year-old patients, the lifetime risks are 63% for reoperation and 83% for bleeding events. For 65-year-old patients, these risks are 28% and 47%, respectively. For 75-year-old patients, these risks are 11% and 24%, respectively. In addition, a separate study found that the mortality risk of reoperation is 3 times less than the mortality risk of a bleeding event.14 These results reflect the greater reoperation risk of implanting a bioprosthetic valve in individuals younger than 60 to 65 years, but illustrate how these reoperation risks may be less significant than bleeding risks associated with anticoagulation.
Surgical factors and co-morbidities
Once the relative advantages and disadvantages of valve type are properly appreciated, then providers need to consider individual patient factors that can influence valve selection. Surgical factors, such as concurrent need for aortic root replacement, may favor one valve type over the other.27 Other co-morbidities, such as atrial fibrillation, renal failure, and other conditions refererred to above, will also affect the valve selection. Other significant medical history of the patient, contraindications, and the potential of pregnancy are other issues that the provider and patient need to consider.
Patient preferences
Once the individual patient characteristics have been considered, discussion should then turn to the patient’s values, anxieties and expectations for their lifestyle and quality of life and how these affect the valve selection. Ultimately, assuming no contraindication to anticoagulants, the patient’s choice will largely depend on which potential outcome, reoperation with a tissue valve or lifelong anticoagulation with a mechanical valve, he or she wishes to keep at a minimum.15 If the patient refuses to be on warfarin therapy regardless of reoperation risk, then the valve type selection process is relatively straightforward. Similarly, if the patient is strongly opposed to reoperation and would rather take life-long warfarin, then the valve type selection process is also relatively straightforward. However, when the patient’s preferences are not strongly associated with one valve type over another, the provider and patient must together evaluate the complex issues of valve selection and make a decision.
Evaluation of competing risks is a complex task, due to the uncertainty surrounding various outcomes, the difficulty in valuing future events, and the relative unfamiliarity patients have with the medical consequences of their decisions. It is essential to communicate these risks in an understandable form to patients and to provide frameworks for decision-making.
Decision aids
Decision aids are a promising approach in effective communication of risks to patients. One such example of their effectiveness has been illustrated among patients with early stage breast cancer and their need to choose between mastectomy and lumpectomy. Decision aids have been constructed to illustrate the options in an understandable format, using pie graphs and other illustrative diagrams. Investigators have studied the effectiveness of decision aids on knowledge in decision-making about breast cancer surgery. They found that decision aids enable the patient to be more knowledgeable about the treatment options, reduce decisional conflict, and ultimately bring the patient more satisfaction of the decision.38 These tools improve the decision-making process by placing an emphasis on patient knowledge and therefore ultimately patient preference. This shared decision-making process offers the patient ownership of their decision, which can lead to better medication and therapeutic regimen adherence. Employing decision aids in prosthetic valve selection may offer similarly beneficial outcomes.
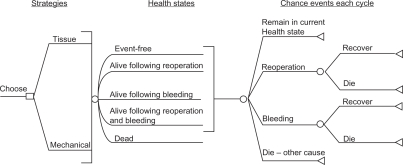
Once risks have been properly specified and communicated, they need to be incorporated into an overall decision-making framework to assist providers and patients in making an optimal choice. Two decision-making models are potentially useful. One option is the utilization of decision trees. Decision trees model current and future choices and calculate the probability of various outcomes. The decision tree is a useful decision-making tool that simplifies complex issues into a more understandable format. In addition, decision trees can incorporate patient preferences in the decision-making process. For example, Figure 7 is a decision tree that associates chance events with each valve selection in a framework that allows for more effective decision-making.26
Figure 7.
Schematic of decision model structure. All patients are initially in the event-free state. With each 1-year cycle of the model, they can move to a different state or remain in the same state according to chance events specified under “Chance events each cycle”.
Notes: Event-free: alive without reoperation or major bleeding; Alive following reoperation: alive following reoperation for prosthetic valve failure; Alive following bleeding: alive following a major (requiring hospitalization or blood transfusion) bleeding event; Dead: dead from any cause. Copyright © 2000. Elsevier. Reprinted with permission from Birkmeyer NJ, Birkmeyer JD, Tosteson AN, et al. Prosthetic valve type for patients undergoing aortic valve replacement: a decision analysis. Ann Thorac Surg. 2000;70:1946–1952.26
Another potential tool is a microsimulation model. A microsimulation model calculates probabilities of events, similar to decision models, but then performs thousands of simulations of patients making various decisions and experiencing outcomes within the model. This analysis allows for these simulated patients to reflect similar ages, medical histories, and values of the patient facing the decision, and thus allows the patient and his or her provider to predict more accurately the likely outcome of their decision.10,39 Both microsimulation models and decision trees are potentially helpful options in the valve type selection process.
Costs
Finally, costs associated with valve selection should be part of the decision-making process. From the patient’s perspective, costs include money spent on co-pays, medication, and other medical care; money spent on lifestyle adaptations; and economic costs such as lost income and missed days at work secondary to medical care and/or complications. Costs incurred by family members or other support and their assistance with transportation and emotional support should also be incorporated into the overall valve selection considerations.
Selection of the ideal valve type for a given patient involves a dizzying array of variables. However, by carefully considering the medical circumstances of the individual patient and incorporating their preferences, ideally with the assistance of decision aids and frameworks such as decision or microsimulation models, the likelihood of making a satisfactory choice increases. A patient’s satisfaction with his or her decision can significantly affect their quality of life and highlights the importance of the prosthetic heart valve choice being a shared decision between the provider’s expertise and the patient’s expectations.40
Future directions
Innovations in prosthetic valve construction, anticoagulation, and medical decision-making will all affect future valve selection. For patients considering mechanical valve replacement, improvements in valve structure may lower thrombotic risk and require lower intensity of anticoagulation.14 New oral anticoagulants, such as dabigatran and rivaroxaban, have a fixed dosing regimen that delivers a more consistent anticoagulant effect that may eliminate the need for frequent monitoring. This improved pharmacokinetic profile also has potential to significantly reduce the associated bleeding risks of anticoagulation with mechanical valves.41 Improvements in tissue valves and implantation technique may reduce structural valve deterioration, thus improving valve durability and reducing reoperation rates. Any or all of these innovations would substantially affect the current considerations in prosthetic valve selection.
Improvements in decision aids and medical decision-making need to accompany technical improvements in prosthetic valves and anticoagulation. Further refinement of risk quantification and communication of risk to patients is essential to improve patient comprehension and the quality of decision-making. An integral part of refinements in risk quantification is ongoing research in the actual outcomes of contemporary patients receiving valve replacements. Many studies that form the foundation of our understanding of valve outcomes are derived from studies occurring decades ago and primarily in Caucasian males. Updating this information with outcomes from a more diverse patient population using modern surgical techniques, valve technology, and anticoagulation strategies will allow for more precise characterization of the risks and benefits that current patients face in valve selection.
Conclusion
Optimal valve selection results when the patient and provider carefully consider the advantages and disadvantages of each valve type in the context of the individual patient’s age, clinical conditions, values, and lifestyle desires. The provider needs to communicate clearly the medical considerations, and both provider and patient need to appropriately weigh medical and individual considerations in the final decision, ideally employing validated decision aids and models to assist with their deliberation. This calculated and thorough approach provides the best opportunity for achieving optimal outcomes in prosthetic valve selection and subsequent replacement.
Acknowledgments
We would like to acknowledge Dr Daniel Matlock for his advice on the use of decision-making aids in the selection of prosthetic heart valves.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jones EC, Devereux RB, Roman MJ, et al. Prevalence and correlates of mitral regurgitation in a population-based sample (the Strong Heart study) Am J Cardiol. 2001;87:298–304. doi: 10.1016/s0002-9149(00)01362-x. [DOI] [PubMed] [Google Scholar]
- 2.Bach DS, Radeva JI, Birnbaum HG, Fournier AA, Tuttle EG. Prevalence, referral patterns, testing, and surgery in aortic valve disease: leaving women and elderly patients behind. J Heart Valve Dis. 2007;16:362–369. [PubMed] [Google Scholar]
- 3.Pibarot P, Dumesnil JG. Prosthetic heart valves: selection of the optimal prosthesis and long-term management. Circulation. 2009;119:1034–1048. doi: 10.1161/CIRCULATIONAHA.108.778886. [DOI] [PubMed] [Google Scholar]
- 4.Vongpatanasin W, Hillis LD, Lange RA. Medical progress prosthetic heart valves. N Engl J Med. 1996;335:407–416. doi: 10.1056/NEJM199608083350607. [DOI] [PubMed] [Google Scholar]
- 5.Masters RG, Helou J, Pipe AL, Keon WJ. Comparative clinical outcomes with St. Jude Medical, Medtronic Hall and CarboMedics mechanical heart valves. J Heart Valve Dis. 2001;10:403–409. [PubMed] [Google Scholar]
- 6.Vongpatanasin W, Hillis LD, Lange RA. Prosthetic heart valves. N Engl J Med. 1996;335:407–416. doi: 10.1056/NEJM199608083350607. [DOI] [PubMed] [Google Scholar]
- 7.Yacoub M, Rasmi NRH, Sundt TM, et al. Fourteen-year experience with homovital homografts for aortic valve replacement. J Thorac Cardiovasc Surg. 1995;60:110–186. doi: 10.1016/S0022-5223(05)80025-X. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien MF, Stafford EG, Gardner MA, et al. Allograft aortic valve replacement: long-term follow-up. Ann Thorac Surg. 1995;60:S65. doi: 10.1016/0003-4975(95)00223-8. [DOI] [PubMed] [Google Scholar]
- 9.Hammermeister KE, Sethi GK, Henderson WG, et al. A comparison of outcomes in men 11 years after heart-valve replacement with a mechanical valve or bioprosthesis. Veterans Affairs Cooperative Study on Valvular Heart Disease. N Engl J Med. 1993;328:1289–1296. doi: 10.1056/NEJM199305063281801. [DOI] [PubMed] [Google Scholar]
- 10.Puvimanasinghe JPA, Steyerberg EW, Takkenberg JJM, Eijkemans EJ, van Herwerden LA. Prognosis after aortic valve replacement with a bioprosthesis: predictions based on meta-analysis and microsimulation. Circulation. 2001;103:1535–1541. doi: 10.1161/01.cir.103.11.1535. [DOI] [PubMed] [Google Scholar]
- 11.Hammermeister K, Sethi GK, Henderson WG, Oprian C, Rahimtoola SH. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol. 2000;36:1152–1158. doi: 10.1016/s0735-1097(00)00834-2. [DOI] [PubMed] [Google Scholar]
- 12.Oxenham H, Bloomfield P, Wheatley DJ, et al. Twenty year comparison of a Bjork-Shiley mechanical heart valve with porcine bioprostheses. Heart. 2003;89:715–721. doi: 10.1136/heart.89.7.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahimtoola SH. Choice of prosthetic heart valve for adult patients. J Am Coll Cardiol. 2003;41:893–904. doi: 10.1016/s0735-1097(02)02965-0. [DOI] [PubMed] [Google Scholar]
- 14.Van Geldorp M, Jamieson E, Kappetein AP, et al. Patient outcome after aortic valve replacement with a mechanical or biological prosthesis: weighing lifetime anticoagulant-related event risk against reoperation risk. J Thorac Cardiovasc Surg. 2009;137:881–886. doi: 10.1016/j.jtcvs.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 15.Rahimtoola SH. Choice of prosthetic heart valve in adults: an update. J Am Coll Cardiol. 2010;55:2413–2426. doi: 10.1016/j.jacc.2009.10.085. [DOI] [PubMed] [Google Scholar]
- 16.The Stroke Prevention in Atrial Fibrillation Investigators Bleeding during antithrombotic therapy in patients with atrial fibrillation. Arch Intern Med. 1996;156:409–416. [PubMed] [Google Scholar]
- 17.Byeth R, Quinn L, Landefeld C. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med. 1998;105:91–99. doi: 10.1016/s0002-9343(98)00198-3. [DOI] [PubMed] [Google Scholar]
- 18.Fihn S, Callahan C, Martin D, McDonell M, Henikoff J, White R. The risk for and severity of bleeding complications in elderly patients treated with warfarin. Ann Intern Med. 1996;124:970–979. doi: 10.7326/0003-4819-124-11-199606010-00004. [DOI] [PubMed] [Google Scholar]
- 19.Landefeld C, Goldman L. Major bleeding in outpatients treated with warfarin: incidence and prediction by factors known at the start of outpatient therapy. Am J Med. 1989;87:144–152. doi: 10.1016/s0002-9343(89)80689-8. [DOI] [PubMed] [Google Scholar]
- 20.Landefeld C, Beyth R. Anticoagulant-related bleeding. Clinical epidemiology, prediction, and prevention. Am J Med. 1993;95:315–328. doi: 10.1016/0002-9343(93)90285-w. [DOI] [PubMed] [Google Scholar]
- 21.Launbjerg J, Egeblad H, Heaf J, Nielsen N, Fuglehom A, Ladefoged K. Bleeding complications to oral anticoagulant therapy: multivariate analysis of 1010 treatment years in 551outpatients. J Intern Med. 1991;229:351–355. doi: 10.1111/j.1365-2796.1991.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 22.Palareti G, Leali N, Coccheri S, et al. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study. Lancet. 1996;348:423–428. doi: 10.1016/s0140-6736(96)01109-9. [DOI] [PubMed] [Google Scholar]
- 23.Palareti G, Manotti C, D’Angelo A, et al. Thrombotic events during oral anticoagulant treatment: results of the inception cohort, prospective, collaborative ISCOAT study. Thromb Haemost. 1997;78:1438–1443. [PubMed] [Google Scholar]
- 24.Steffensen F, Kristensen K, Ejlersen E, Dahlerup J, Sorensen H. Major haemorrhagic complications during oral anticoagulant therapy in a Danish population-based cohort. J Intern Med. 1997;242:497–503. doi: 10.1111/j.1365-2796.1997.tb00023.x. [DOI] [PubMed] [Google Scholar]
- 25.Van der Meer F, Rosendaal F, Vandenbroucke J, Briet E. Assessment of a bleeding risk index in two cohorts of patients treated with oral anticoagulants. Thromb Haesmost. 1996;76:12–16. [PubMed] [Google Scholar]
- 26.Birkmeyer NJ, Birkmeyer JD, Tosteson AN, et al. Prosthetic valve type for patients undergoing aortic valve replacement: a decision analysis. Ann Thorac Surg. 2000;70:1946–1952. doi: 10.1016/s0003-4975(00)01863-4. [DOI] [PubMed] [Google Scholar]
- 27.De Vincentiis C, Kunkl AB, Trimarchi S, et al. Aortic valve replacement in octogenarians: is biologic valve the unique solution. Ann Thorac Surg. 2008;85:1296–1301. doi: 10.1016/j.athoracsur.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 28.Hung L, Rahimtoola SH. Prosthetic heart valves and pregnancy. Circulation. 2003;107:1240–1246. doi: 10.1161/01.cir.0000060806.86686.ec. [DOI] [PubMed] [Google Scholar]
- 29.Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006 Aug 1;114:e84–e231. doi: 10.1161/CIRCULATIONAHA.106.176857. [DOI] [PubMed] [Google Scholar]
- 30.Vahanian A, Baumgartner H, Bax J, et al. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J. 2007;28:230–268. doi: 10.1093/eurheartj/ehl428. [DOI] [PubMed] [Google Scholar]
- 31.Chan V, Jamieson WR, Germann E, et al. Performance of bioprostheses and mechanical prostheses assessed by composites of valve-related complications to 15 years after aortic valve replacement. J Thorac Cardiovasc Surg. 2006;131:1267–1273. doi: 10.1016/j.jtcvs.2005.11.052. [DOI] [PubMed] [Google Scholar]
- 32.Kappetein AP, Takkenberg J, Puvimanasinghe J, Jamieson WR, Eijkemans M, Bogers A. Does the type of biological valve affect patient outcome. Interact Cardiovasc Thorac Surg. 2006;5:398–402. doi: 10.1510/icvts.2005.122382. [DOI] [PubMed] [Google Scholar]
- 33.Kulik A, Masters RG, Bédard P, et al. Postoperative lipid-lowering therapy and bioprosthesis structural valve deterioration: justification for a randomised trial. Eur J Cardiothorac Surg. 2010;37:139–144. doi: 10.1016/j.ejcts.2009.06.051. [DOI] [PubMed] [Google Scholar]
- 34.Carpentier A. Hemodynamic factors affecting the fate of valvular bioprosthesis. Circulation. 2010;121:2083–2084. doi: 10.1161/CIRCULATIONAHA.110.954123. [DOI] [PubMed] [Google Scholar]
- 35.Kvidal P, Bergstrom R, Horte LG, Stable E. Observed and relative survival after aortic valve replacement. J Am Coll Cardiol. 2000;35:747–756. doi: 10.1016/s0735-1097(99)00584-7. [DOI] [PubMed] [Google Scholar]
- 36.Puvimanasinghe JPA, Takkenberg JJM, Edwards M, et al. Comparison of outcomes after aortic valve replacement with a mechanical valve or a bioprosthesis using microsimulation. Heart. 2004;90:1172–1178. doi: 10.1136/hrt.2003.013102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grunkemeier G, Jamieson W, Miller D, Starr A. Actuarial versus actual risk of porcine structural valve deterioration. J Thorac Cardiovasc Surg. 1994;108:709–718. [PubMed] [Google Scholar]
- 38.Whelan T, Levine M, Willan A, et al. Effect of a decision aid on knowledge and treatment decision making for breast cancer surgery: a randomized trial. JAMA. 2004;292:435–441. doi: 10.1001/jama.292.4.435. [DOI] [PubMed] [Google Scholar]
- 39.Takkenberg JJM, Puvimanasinghe JPA, van Herwerden LA. Decision-making in aortic valve replacement: bileaflet mechanical valves versus stented bioprostheses. Neth Heart J. 2003;11:5–10. [PMC free article] [PubMed] [Google Scholar]
- 40.Cher D, Miyamoto J, Lenert L. Incorporating risk attitude into Markov-process decision models: importance for individual decision making. Med Decis Making. 1997;17:340–350. doi: 10.1177/0272989X9701700311. [DOI] [PubMed] [Google Scholar]
- 41.Borris LC. Rivaroxaban and dabigatran etexilate: two new oral anticoagulants for extended postoperative prevention of venous thromboembolism after elective total hip arthroplasty. Arch Orthop Trauma Surg. 2010;130:583–589. doi: 10.1007/s00402-009-0930-9. [DOI] [PubMed] [Google Scholar]