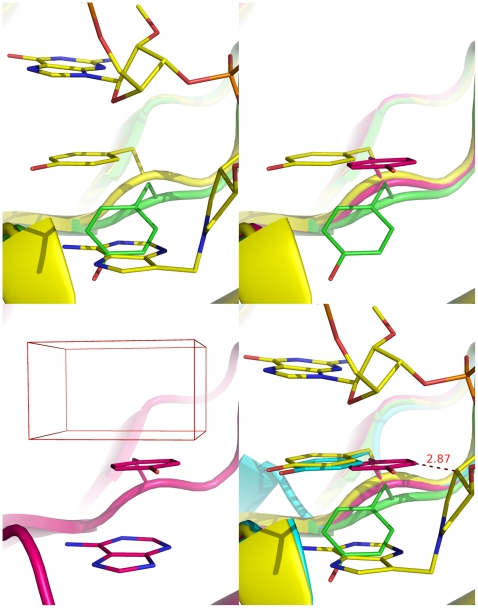
Figure 2. Tyr80 in crystal structures of ricin subunit A (RTA) in the bound and unbound states.
a (top left): overlay of the apo RTA (green, 1IFT [32]) with the oligonucleotide-bound RTA at the Michaelis-Menten state (yellow; 3HIO [13]) showing that the adenine group markedly perturbs the conformation of Tyr80; b (top right): three distinct conformations of Tyr80: conformations 1, 2, and 3 represent the apo conformation in green (1IFT [32]), the less populated bound conformation in magenta (1IFS [32]), and the most populated bound conformation in yellow (1FMP [31]), respectively; c (bottom left): the phenolic ring with an adenine group underneath and a docking box atop in the less populated bound conformation (1IFS [32]); d (bottom right): overlay of the oligonucleotide-bound RTA at the Michaelis-Menten state (yellow; 3HIO [13]) with RTA in conformation 1 (green; 1IFT [32]), conformation 2 (magenta; 1IFS [32]), and conformation 3 (cyan; 1FMP [31]) showing the closeness of the Tyr80 conformations in 3HIO and 1FMP and the clash between the nucleotide and Tyr80 in 1IFS.