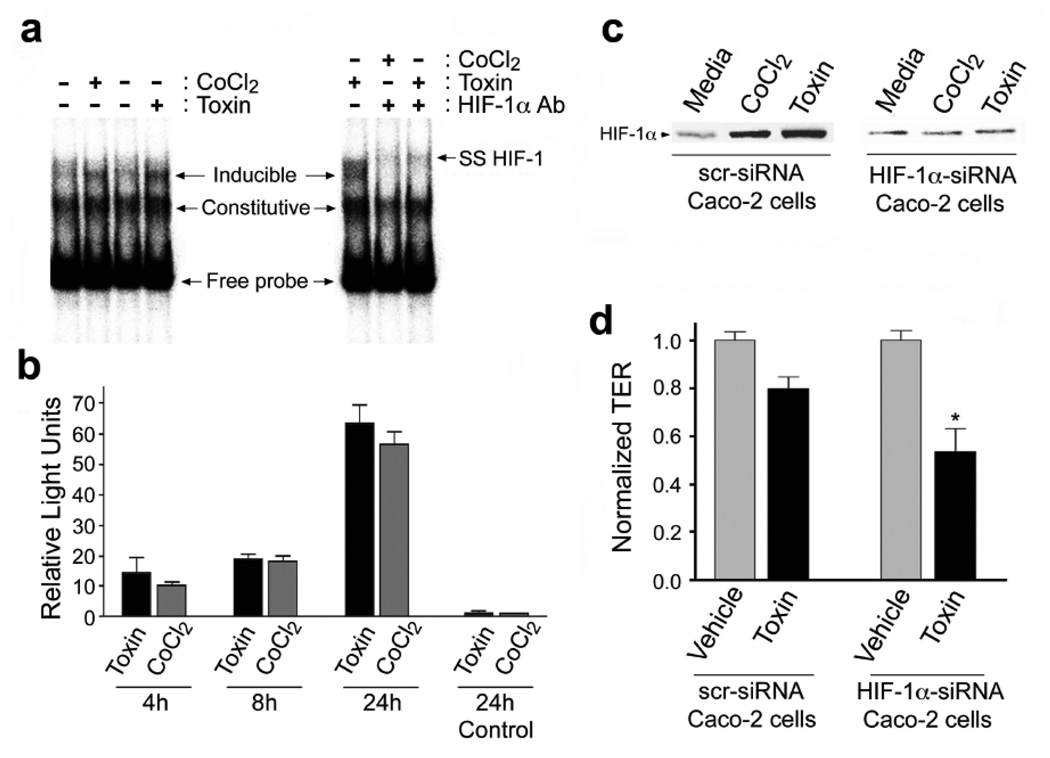
Figure 3.
C.difficile toxin exposure promotes HIF-1 dependent promoter binding activity and transcriptional activity in Caco-2 cells. (a) DNA binding of HIF-1α to the EPO hypoxia response element (HRE) was assessed by electrophoretic mobility shift assay (EMSA). Nuclear extracts were prepared from Caco-2 cells that had been exposed to C.difficile toxin (100µg/mL), or CoCl2 (100µM). In some cases, EMSA “supershifts” were generated with addition of anti-HIF-1α monoclonal antibody to the DNA binding reactions. (b) Toxin exposure promotes HIF-1α transcriptional activity in Caco-2 cells as measured by a luciferase reporter assay. Caco-2 cells were transiently co-transfected with firefly luciferase reporter and Renilla luciferase control plasmids. Cells were incubated for 4, 18 or 24h under normoxic conditions with 10µg/mL C.difficile toxin or 100µM CoCl2. The transfection efficiency was corrected by normalizing firefly luciferase activity to the Renilla luciferase activity. Control cells were transfected with empty pGL3 vector and treated for 24h with C.difficile toxin or CoCl2. All values (mean±S.E.M., n=4) are expressed as fold-induction relative to the controls that were arbitrarily defined as 1. (c–d) Caco-2 cells deficient in HIF-1α are more susceptible to C.difficile toxin-induced injury. (c) HIF-1α protein levels in Caco-2 cells stably transfected with HIF-1α siRNA (Caco-2 HIF-1α−/−) were not affected by C.difficile toxin or CoCl2. (d) Caco-2 HIF-1α−/− cells display increased susceptibility to C.difficile toxin, as assessed by transepithelial resistance (TER) following a 2h incubation with toxin (60µg/mL). All values are mean ± S.E.M., (n=4), * denotes p<0.05.