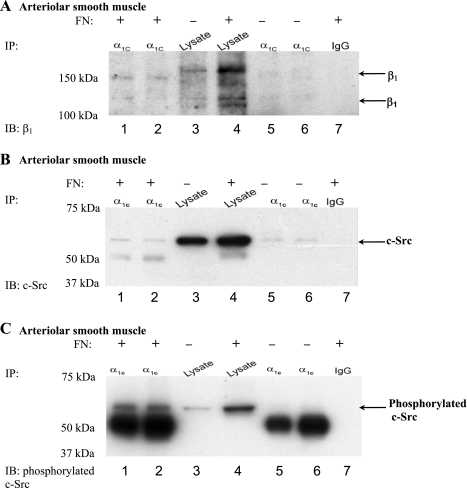
Fig. 3.
α1C-CaL associates with β1-integrin in arteriolar smooth muscle incubated with FN. A: representative blot showing co-IP protocol on protein lysed from arteriolar smooth muscle (pulled down with anti-α1C-CaL). β1-Integrin and α1C-CaL coimmunoprecipitated when arterioles were incubated with FN for 1 h (lanes 1 and 2, respectively). Lanes 1 and 6 were immunoprecipitated with anti-α1C-CaL from Chemicon, whereas lanes 2 and 5 were immunoprecipitated with anti-α1C-CaL from Abcam. Lanes 3 and 4 were lysates alone isolated from control arterioles or arterioles incubated with FN, respectively. The absence of coimmunoprecipitated β1-integrin by rabbit IgG (lane 7) indicates binding specificity. B: representative blot showing co-IP protocol on protein lysed from arteriolar smooth muscle (pulled down with anti-α1C-CaL). c-Src coimmunoprecipitated with α1C-CaL whether or not arterioles were incubated with FN (lanes 1 and 2 vs. lanes 5 and 6, respectively). IP was performed as described in A. Abluminal incubation of arterioles with exogenous FN did not change the degree of association between α1C-CaL and c-Src (lane 2, 90% and lane 5, 86% of arterioles alone, respectively). The absence of coimmunoprecipitated c-Src by rabbit IgG (lane 7) indicates binding specificity. C: representative blot showing co-IP protocol on protein lysed from arteriolar smooth muscle (pulled down with anti-α1C-CaL). Phosphorylated c-Src was only observed in arterioles incubated with exogenous FN (lanes 1 and 2). IP was performed as described in A. The absence of phosphorylated c-Src by rabbit IgG (lane 7) indicates binding specificity.