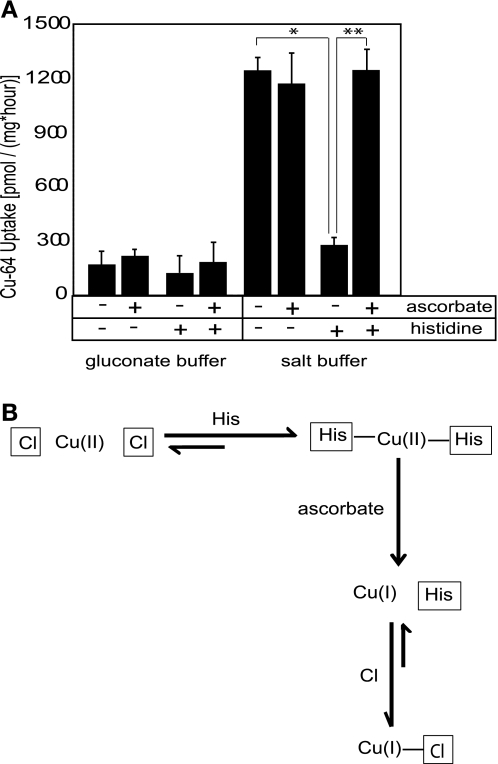
Fig. 4.
The role of the oxidation state of copper. A: ascorbate reverses histidine inhibition of chloride-dependent copper uptake in Caco2 cells. Cu uptakes in Transwells were determined in either salt transport buffer (150 mM NaCl, 5 mM KCl, 2.5 mM MgCl2, 25 mM HEPES, pH 7.4) or chloride-free gluconate buffer (150 mM Na-gluconate, 5 mM K-gluconate, 25 mM HEPES, pH 7.4). Histidine (100 μM) and/or ascorbate (1 mM) was included in the apical compartment of Transwells as indicated in the table. Values are shown as an average ± SD at each point. Two-tailed independent Student's t-test was performed; *P < 0.005 for control vs. histidine-supplemented buffer; ** P < 0.005 for histidine vs. histidine plus ascorbate buffer. B: mechanism of reversal of histidine inhibition by ascorbate. Histidine (pKa 6.0) when placed in solution at pH 7.4 is deprotonated and will compete with chloride ions to form a His2Cu complex with copper (II) via its imidazole nitrogen. Once the copper-histidine complex is formed, it cannot be transported into the cell as a pseudo-substrate of the anion exchanger, which results in an inhibition of copper uptake. However, when copper is reduced by ascorbate, Cu(I)-histidine complexes are less stable than Cu(II)-histidine complexes, and chloride ions, present at ∼150 mM in physiological solution, will compete successfully with histidine for Cu(I). Cu(I)-chloride complexes can enter the cell via the anion exchange mechanism, which results in stimulation of copper uptake (i.e., apparent reversal of histidine inhibition).