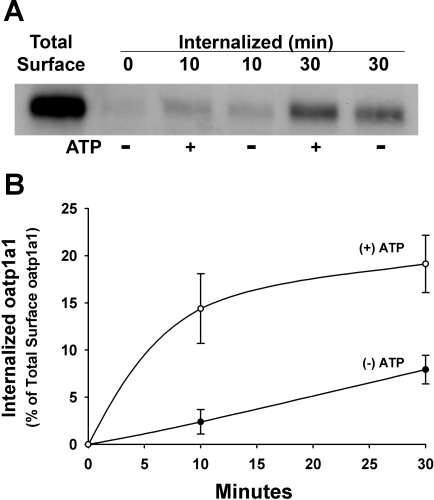
Fig. 7.
Influence of phosphorylation on internalization of cell surface oatp1a1 in overnight-cultured rat hepatocytes. Hepatocytes were isolated from rat liver and cultured overnight, surface biotinylated with membrane-impermeant sulfo-NHS-SS-biotin for 30 min at 4°C, and then incubated at 37°C for 10 or 30 min in the absence (−) or presence (+) of 1 mM ATP. Previous studies showed that this short incubation of rat hepatocytes in extracellular ATP stimulates serine phosphorylation of oatp1a1 via activity of a purinergic receptor. After removal of residual biotin from the cell surface by reduction, internalized biotinylated oatp1a1 was collected on streptavidin-agarose beads and subjected to immunoblot for oatp1a1. A: representative study. B: densitometric quantitation of 3 experiments. Data were normalized to total starting cell surface biotinylated oatp1a1. Values are means ± SE.