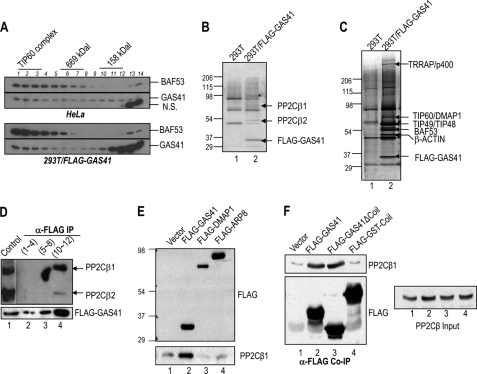
FIGURE 1.
A small GAS41 complex copurifies with two isoforms of human PP2Cβ. A, immunoblots of BAF53 and GAS41, which were resolved by Sepharose CL6B chromatography. Nuclear extracts from HeLa (upper panel) and 293T/FLAG-GAS41 (bottom panel) cells were analyzed with Sepharose CL6B gel filtration. Higher numbers in lanes represent lower molecular masses. N.S., not significant. B, SDS-PAGE/silver stain analysis of mock control and FLAG-GAS41 preparations from the Sepharose CL6B gel filtration analysis (A, lower panel, lanes 10–12) followed by affinity purification on M2 agarose. A nonspecific contaminant band is marked by the asterisk. C, SDS-PAGE/silver stain analysis of mock control and FLAG-GAS41 preparations from the Sepharose CL6B gel filtration analysis (A, lower panel, lanes 1–3) followed by affinity purification on M2 agarose. D, coimmunoprecipitation (IP) of PP2Cβ with FLAG-GAS41. Three pooled fractions (fractions 1–4, 5–8, and 10–12), originally resolved by Sepharose CL6B gel filtration (A, bottom panel), were subject to immunoprecipitation by M2 agarose. FLAG-GAS41 and enrichment of endogenous PP2Cβ1 and PP2Cβ2 were analyzed by immunoblot with anti-FLAG and anti-PP2Cβ antibodies. HeLa nuclear extract was used as a control (lane 1). E, selective association of PP2Cβ1 with GAS41 but not with a subunit of chromatin-remodeling complexes in 293T cells. FLAG-tagged GAS41, DMAP1, and ARP8 were ectopically expressed in 293T cells and subjected to immunoprecipitation using M2 agarose. F, interaction of PP2Cβ1 with the YEATS domain of GAS41. FLAG-tagged GAS41 (1–225), FLAG-GAS41ΔCoil (C-terminal-deleted GAS41 (1–192)), and FLAG-GST-Coil (GAS41 C-terminal domain fused with GST) were ectopically expressed in 293T cells and subjected to immunoprecipitation by M2 agarose.