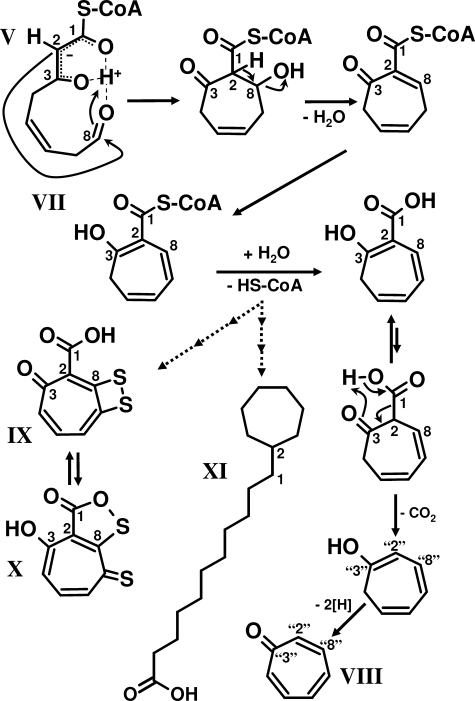
FIGURE 4.
Proposed mechanism of 2-hydroxycyclohepta-1,4,6-triene-1-formyl-CoA formation. The highly reactive 3-oxo-5,6-dehydrosuberyl-CoA semialdehyde (V, ring-cleavage product) undergoes spontaneous intramolecular Knoevenagel-type condensation forming the derivative 2-hydroxycyclohepta-1,4,6-triene-1-formyl-CoA (VII). The aromatic tropone (IX) is likely formed from VII, whereas the antibiotics tropodithietic acid (IX) and thiotropocin (X) as well as the ω-cycloheptyl fatty acids (XI) are derived either directly from VII or from the same compound after hydrolysis of the CoA thioester. Note that the numbering of C-atoms for the cyclic compounds is not according to nomenclature.